question_answer 1) In an experiment four quantities a, b, c and d are measured with percentage error 1%, 2%, 3% and 4% respectively. Quantity P is calculated as follows \[p=\frac{{{a}^{3}}{{b}^{2}}}{cd}%,\] Error in P is
A)
14%
done
clear
B)
10%
done
clear
C)
7%
done
clear
D)
4%
done
clear
View Answer play_arrow
question_answer 2)
The velocity of a projectile at the initial point A is (2i + 3j) m/s. Its velocity (in m/s) at point B is
A)
-2i-3j
done
clear
B)
-2i+3j
done
clear
C)
2i-3j
done
clear
D)
2i+3j
done
clear
View Answer play_arrow
question_answer 3) A stone falls freely under gravity. It covers distances \[{{h}_{1}},{{h}_{2}}\] and \[{{h}_{3}}\], in the first 5 seconds, the next 5 seconds and the next 5 seconds respectively. The relation between \[{{h}_{1}},{{h}_{2}}\] and \[{{h}_{3}}\] is
A)
\[{{h}_{1}}=2{{h}_{2}}=3{{h}_{3}}\]
done
clear
B)
\[{{h}_{1}}=\frac{{{h}_{2}}}{3}=\frac{{{h}_{3}}}{5}\]
done
clear
C)
\[{{h}_{2}}=3{{h}_{1}}\]and\[{{h}_{3}}=3{{h}_{2}}\]
done
clear
D)
\[{{h}_{1}}={{h}_{2}}={{h}_{3}}\]
done
clear
View Answer play_arrow
question_answer 4)
Three blocks with masses m, 2m and 3m are connected by strings, as shown in the figure. After an upward force is applied on block m, the masses move upward at constant speed v. What is the net force on the block of mass 2m? (g is the acceleration due to gravity)
A)
Zero
done
clear
B)
2mg
done
clear
C)
3mg
done
clear
D)
6mg
done
clear
View Answer play_arrow
question_answer 5) The upper half of an inclined plane of inclination 9 is perfectly smooth while lower half is rough. A block starting from rest at the top of the plane will again come to rest at the bottom, if the coefficient of friction between the block and lower half of the plane is given by
A)
\[\mu =\frac{1}{\tan \theta }\]
done
clear
B)
\[\mu =\frac{2}{\tan \theta }\]
done
clear
C)
\[\mu =2\tan \theta \]
done
clear
D)
\[\mu =\tan \theta \]
done
clear
View Answer play_arrow
question_answer 6) A uniform force of \[(3\mathbf{i}+\mathbf{j})\] N acts on a particle of mass 2 kg. Hence the particle is displaced from position \[(2\mathbf{i}+\mathbf{k})\] m to position \[(4\mathbf{i}+3\mathbf{j}-\mathbf{k})\] m. The work done by the force on the particle is
A)
9 J
done
clear
B)
6 J
done
clear
C)
13 J
done
clear
D)
15 J
done
clear
View Answer play_arrow
question_answer 7) An explosion breaks a rock into three parts in a horizontal plane. Two of them go off at right angles to each other. The first part of mass 1 kg moves with a speed of 12 ms-1 and the second part of mass 2 kg moves with 8 ms-1 speed. If the third part flies off with 4 ms-1 speed, then its mass is
A)
3 kg
done
clear
B)
5 kg
done
clear
C)
7kg
done
clear
D)
17kg
done
clear
View Answer play_arrow
question_answer 8)
A rod PQ of mass M and length L is hinged at end P. The rod is kepts horizontal by a massless string tied to point Q as shown in figure. When string is cut, the initial angular acceleration of the rod is
A)
\[\frac{3g}{2L}\]
done
clear
B)
\[\frac{g}{L}\]
done
clear
C)
\[\frac{2g}{L}\]
done
clear
D)
\[\frac{2g}{3L}\]
done
clear
View Answer play_arrow
question_answer 9) A small object of uniform density rolls up a curved surface with an initial velocity v'. It reaches up to a maximum height of \[\frac{3{{\upsilon }^{2}}}{4g}\]with respect to the initial position. The object is
A)
ring
done
clear
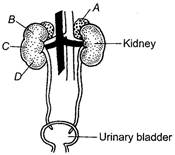
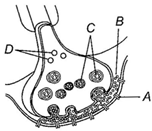
B)
solid sphere
done
clear
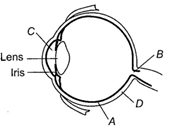
C)
hollow sphere
done
clear
D)
disc
done
clear
View Answer play_arrow
question_answer 10) A body of mass m taken from the earth's surface to the height equal to twice the radius (R) of the earth. The change is potential energy of body will be
A)
\[mg2R\]
done
clear
B)
\[\frac{2}{3}mgR\]
done
clear
C)
\[3mgR\]
done
clear
D)
\[\frac{1}{3}mgR\]
done
clear
View Answer play_arrow
question_answer 11) Infinite number of bodies, each of mass 2 kg are situated on x-axis at distance 1m, 2 m, 4 m, 8 m, respectively from the origin. The resulting gravitational potential due to this system at the origin will be
A)
\[-G\]
done
clear
B)
\[-\frac{8}{3}G\]
done
clear
C)
\[-\frac{4}{3}G\]
done
clear
D)
\[-4G\]
done
clear
View Answer play_arrow
question_answer 12) The following four wires are made of the same material. Which of these will have the largest extension when the same tension is applied?
A)
Length = 50 cm, diameter = 0.5 mm
done
clear
B)
Length = 100 cm, diameter = 1 mm
done
clear
C)
Length = 200 cm, diameter = 2 mm
done
clear
D)
Length = 300 cm, diameter = 3 mm
done
clear
View Answer play_arrow
question_answer 13) The wet ability of a surface by a liquid depends primarily on
A)
viscosity
done
clear
B)
surface tension
done
clear
C)
density
done
clear
D)
angle of contact between the surface and the liquid
done
clear
View Answer play_arrow
question_answer 14) The molar specific heats of an ideal gas at constant pressure and volume are denoted by \[{{C}_{p}}\] and \[{{C}_{V}}\] respectively. If \[\gamma =\frac{{{C}_{p}}}{{{C}_{V}}}\] and R is the universal gas constant, then \[{{C}_{V}}\] is equal to
A)
\[\frac{1+\gamma }{1-\gamma }\]
done
clear
B)
\[\frac{R}{(\gamma -1)}\]
done
clear
C)
\[\frac{(\gamma -1)}{R}\]
done
clear
D)
\[\gamma R\]
done
clear
View Answer play_arrow
question_answer 15) A piece of iron is heated in a flame. If first becomes dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using
A)
Stefan's law
done
clear
B)
Wien's displacement law
done
clear
C)
Kirchoff's law
done
clear
D)
Newton's law of cooling
done
clear
View Answer play_arrow
question_answer 16)
A gas is taken through the cycle \[A\to B\to C\to A,\] as shown. What is the net work done by the gas?
A)
2000 J
done
clear
B)
1000 J
done
clear
C)
Zero
done
clear
D)
-2000 J
done
clear
View Answer play_arrow
question_answer 17) During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of \[\frac{{{C}_{p}}}{{{C}_{\upsilon }}}\] for the gas is
A)
\[\frac{4}{3}\]
done
clear
B)
2
done
clear
C)
\[\frac{5}{3}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 18)
In the given (V-T) diagram, what is the relation between pressures \[{{p}_{1}}\] and \[{{p}_{2}}\] ?
A)
\[{{p}_{2}}={{p}_{1}}\]
done
clear
B)
\[{{p}_{2}}>{{p}_{1}}\]
done
clear
C)
\[{{p}_{2}}<{{p}_{1}}\]
done
clear
D)
Cannot be predicted
done
clear
View Answer play_arrow
question_answer 19) The amount of heat energy required to raise the temperature of 1 g of helium at NTP, from \[{{T}_{1}}K\]To \[{{T}_{2}}K\]is
A)
\[\frac{3}{8}{{N}_{a}}{{K}_{B}}({{T}_{2}}-{{T}_{1}})\]
done
clear
B)
\[\frac{3}{2}{{N}_{a}}{{K}_{B}}({{T}_{2}}-{{T}_{1}})\]
done
clear
C)
\[\frac{3}{4}{{N}_{a}}{{K}_{B}}({{T}_{2}}-{{T}_{1}})\]
done
clear
D)
\[\frac{3}{4}{{N}_{a}}{{K}_{B}}\left( \frac{{{T}_{2}}}{{{T}_{1}}} \right)\]
done
clear
View Answer play_arrow
question_answer 20) A wave travelling in the positive a-direction having displacement along .y-direction as 1 m, wavelength\[2\pi m\]and frequency of \[\frac{1}{\pi }\]Hz is represented by
A)
\[y=\sin (x-2t)\]
done
clear
B)
\[y=\sin (2\pi x-2\pi t)\]
done
clear
C)
\[y=\sin (10\pi x-20\pi t)\]
done
clear
D)
\[y=\sin (2\pi x+2\pi t)\]
done
clear
View Answer play_arrow
question_answer 21) If we study the vibration of a pipe open at both ends, then the following statements is not true
A)
Open end will be anti-node
done
clear
B)
Odd harmonics of the fundamental frequency will be generated
done
clear
C)
All harmonics of the fundamental frequency will be generated
done
clear
D)
Pressure change will be maximum at both ends
done
clear
View Answer play_arrow
question_answer 22) A source of unknown frequency gives 4 beats/s when sounded with a source of known frequency 250 Hz. The second harmonic of the source of unknown frequency gives five beats per second when sounded with a source of frequency 513 Hz. The unknown frequency is
A)
254 Hz
done
clear
B)
246 Hz
done
clear
C)
240 Hz
done
clear
D)
260 Hz
done
clear
View Answer play_arrow
question_answer 23)
Two pith balls carrying equal charges are suspended from a common point by strings of equal length, the equilibrium separation between them is r. Now the strings are rigidly clamped at half the height. The equilibrium separation between the balls now become.
A)
\[{{\left( \frac{1}{\sqrt{2}} \right)}^{2}}\]
done
clear
B)
\[\left( \frac{r}{\sqrt[3]{2}} \right)\]
done
clear
C)
\[\left( \frac{2r}{\sqrt{3}} \right)\]
done
clear
D)
\[\left( \frac{2r}{3} \right)\]
done
clear
View Answer play_arrow
A)
maximum at A
done
clear
B)
maximum at B
done
clear
C)
maximum at C
done
clear
D)
same at all the three points A, B and C A
done
clear
View Answer play_arrow
question_answer 25) A wire of resistance \[4\,\Omega \] is stretched to twice its original length. The resistance of stretched wire would be
A)
\[2\,\Omega \]
done
clear
B)
\[4\,\Omega \]
done
clear
C)
\[8\,\Omega \]
done
clear
D)
\[16\,\Omega \]
done
clear
View Answer play_arrow
question_answer 26) The internal resistance of a 2.1 V cell which gives a current of 0.2 A through a resistance of \[10\,\Omega \] is
A)
\[0.2\,\Omega \]
done
clear
B)
\[0.5\,\Omega \]
done
clear
C)
\[0.8\,\Omega \]
done
clear
D)
\[1.0\,\Omega \]
done
clear
View Answer play_arrow
question_answer 27) The resistances of the four arms P, Q, R and S in a Wheat stone's bridge are \[10\,\Omega ,30\,\Omega ,30\,\Omega \]and \[90\,\Omega ,\] respectively. The emf and internal resistance of the cell are 7 V and \[5\,\Omega \] respectively. If the galvanometer resistance is \[50\,\Omega ,\] the current drawn from the cell will be
A)
1.0 A
done
clear
B)
0.2 A
done
clear
C)
0.1 A
done
clear
D)
2.0 A
done
clear
View Answer play_arrow
question_answer 28) When a proton is released from rest in a room, it starts with an initial acceleration \[{{a}_{0}}\] towards west. When it is projected towards north with a speed \[{{\upsilon }_{0}}\] it moves with an initial acceleration\[3{{a}_{0}}\]towards west. The electric and magnetic fields in the room are
A)
\[\frac{m{{a}_{0}}}{e}west,\frac{2m{{a}_{0}}}{e{{v}_{0}}}up\]
done
clear
B)
\[\frac{m{{a}_{0}}}{e}west,\frac{2m{{a}_{0}}}{e{{v}_{0}}}dwon\]
done
clear
C)
\[\frac{m{{a}_{0}}}{e}east,\frac{3m{{a}_{0}}}{e{{v}_{0}}}up\]
done
clear
D)
\[\frac{m{{a}_{0}}}{e}east,\frac{3m{{a}_{0}}}{e{{v}_{0}}}down\]
done
clear
View Answer play_arrow
question_answer 29) A current loop in a magnetic field
A)
experiences a torque whether the field is uniform or non-uniform in all orientations
done
clear
B)
can be in equilibrium in one orientations
done
clear
C)
can be equilibrium in two orientations, both the equilibrium states are unstable
done
clear
D)
can be in equilibrium in two orientations, one stable while the other is unstable
done
clear
View Answer play_arrow
question_answer 30)
A bar magnet of length I and magnetic dipole moment M is bent in the form of an arc as shown in figure. The new magnetic dipole moment will be
A)
M
done
clear
B)
\[\frac{3}{\pi }M\]
done
clear
C)
\[\frac{2}{\pi }M\]
done
clear
D)
\[\frac{M}{2}\]
done
clear
View Answer play_arrow
question_answer 31) A wire loop is rotated in a magnetic field. The frequency of change of direction of the induced emf is
A)
once per revolution
done
clear
B)
twice per revolution
done
clear
C)
four times per revolution
done
clear
D)
six times per revolution
done
clear
View Answer play_arrow
question_answer 32) A coil of self-inductance L is connected in series with a bulb -B and an AC source. Brightness of the bulb decreases when
A)
frequency of the AC source is decreased
done
clear
B)
number of turns in the coil is reduced
done
clear
C)
a capacitance of reactance \[{{X}_{C}}={{X}_{L}}\] is included in the same circuit
done
clear
D)
an iron rod is inserted in the coil
done
clear
View Answer play_arrow
question_answer 33) The condition under which a microwave oven heats up a food item containing water molecules most efficiently is
A)
the frequency of the microwave must match the resonant frequency of the water molecules
done
clear
B)
the frequency of the microwave has no relation with natural frequency of water molecules
done
clear
C)
microwave are heat waves, so always produce heating
done
clear
D)
infrared waves produce heating in a microwave oven
done
clear
View Answer play_arrow
question_answer 34) Ratio of longest wavelengths corresponding to Lyman and Bulmer series in hydrogen spectrum is
A)
\[\frac{5}{27}\]
done
clear
B)
\[\frac{3}{23}\]
done
clear
C)
\[\frac{7}{29}\]
done
clear
D)
\[\frac{9}{31}\]
done
clear
View Answer play_arrow
question_answer 35) The half-life of a radioactive isotope X is 20 yr. It decays to another element Y which is stable. The two elements X and Y were found to be in the ratio 1:7 in a sample of a given rock. The age of the rock is estimated to be
A)
40 yr
done
clear
B)
60 yr
done
clear
C)
80 yr
done
clear
D)
100 yr
done
clear
View Answer play_arrow
question_answer 36) A certain mass of hydrogen is changed to helium by the process of fusion. The mass defect in fusion reaction is 0.02866 u. The energy liberated per u is (given 1 u = 931 MeV)
A)
2.67 MeV
done
clear
B)
26.7 MeV
done
clear
C)
6.675 MeV
done
clear
D)
13.35 MeV
done
clear
View Answer play_arrow
question_answer 37) For photoelectric emission from certain metal the cut-off frequency is v. If radiation of frequency 2v impinges on the metal plate, the maximum possible velocity of the emitted electron will be (m is the electron mass)
A)
\[\sqrt{\frac{hv}{(2m)}}\]
done
clear
B)
\[\sqrt{\frac{hv}{m}}\]
done
clear
C)
\[\sqrt{\frac{2hv}{m}}\]
done
clear
D)
\[\sqrt[2]{\frac{hv}{m}}\]
done
clear
View Answer play_arrow
question_answer 38) The wavelength \[{{\lambda }_{e}}\]of an electron and \[{{\lambda }_{p}}\] of a photon of same energy E are related by
A)
\[{{\lambda }_{p}}\propto \lambda _{e}^{2}\]
done
clear
B)
\[{{\lambda }_{p}}\propto {{\lambda }_{e}}\]
done
clear
C)
\[{{\lambda }_{p}}\propto \sqrt{{{\lambda }_{e}}}\]
done
clear
D)
\[{{\lambda }_{p}}\propto \frac{1}{\sqrt{{{\lambda }_{e}}}}\]
done
clear
View Answer play_arrow
question_answer 39) A plano-convex lens fits exactly into a plano-concave lens. Their plane surfaces are parallel to each other. If lenses are made of different materials of refractive indices \[{{\mu }_{1}}\] and \[{{\mu }_{2}}\] and -R is the radius of curvature of the curved surface of the lenses, then the focal length of the combination is
A)
\[\frac{R}{2({{\mu }_{1}}+{{\mu }_{2}})}\]
done
clear
B)
\[\frac{R}{2({{\mu }_{1}}-{{\mu }_{2}})}\]
done
clear
C)
\[\frac{R}{({{\mu }_{1}}-{{\mu }_{2}})}\]
done
clear
D)
\[\frac{2R}{({{\mu }_{2}}-{{\mu }_{1}})}\]
done
clear
View Answer play_arrow
question_answer 40) For a normal eye, the cornea of eye provides a converging power of 40 D and the least converging power of the eye lens behind the cornea is 20 D. Using this information, the distance between the retina and the cornea-eye lens can be estimated to be
A)
5 cm
done
clear
B)
2.5 cm
done
clear
C)
1.67cm
done
clear
D)
1.5cm
done
clear
View Answer play_arrow
question_answer 41) In Young's double slit experiment, the slits are 2 mm apart and are illuminated by photons of two wavelengths \[{{\lambda }_{1}}=12000\overset{\text{o}}{\mathop{\,\text{A}}}\,\] and \[{{\lambda }_{2}}=10000\overset{\text{o}}{\mathop{\,\text{A}}}\,\]. At what minimum distance from the common central bright fringe on the screen 2 m from the slit will a bright fringe from one interference pattern coincide with a bright fringe from the other?
A)
8 mm
done
clear
B)
6 mm
done
clear
C)
4 mm
done
clear
D)
3 mm
done
clear
View Answer play_arrow
question_answer 42) A parallel beam of fast moving electrons is incident normally on a narrow slit. A fluorescent screen is placed at a large distance from the slit. If the speed of the electrons is increased, then which of the following statements is correct?
A)
Diffraction pattern is not observed on the screen in the case of electrons
done
clear
B)
The angular width of the central maximum of the diffraction pattern will increase
done
clear
C)
The angular width of the central maximum will decrease
done
clear
D)
The angular width of the central maximum will be unaffected
done
clear
View Answer play_arrow
question_answer 43) In a n-type semiconductor, which of the following statement is true?
A)
Electrons are majority carriers and trivalent atoms are dopants
done
clear
B)
Electrons are minority carriers and pentavalent atoms are dopants
done
clear
C)
Holes are minority carriers and pentavalen atoms are dopants
done
clear
D)
Holes are majority carriers and trivalent atoms are dopants
done
clear
View Answer play_arrow
question_answer 44) In a common emitter (CE) amplifier having a voltage gain G, the transistor used has trans conductance 0.03 mho and current gain 25. If the above transistor is replaced with another one with trans conductance 0.02 mho and current gain 20, the voltage gain will
A)
\[\frac{2}{3}G\]
done
clear
B)
\[1.5G\]
done
clear
C)
\[\frac{1}{3}G\]
done
clear
D)
\[\frac{5}{4}G\]
done
clear
View Answer play_arrow
question_answer 45)
The output (X) of the logic circuit shown in figure will be
A)
\[X=\overline{\overline{A}}\cdot \overline{\overline{B}}\]
done
clear
B)
\[X=\overline{A\cdot B}\]
done
clear
C)
\[X=A\cdot B\]
done
clear
D)
\[X=\overline{A+B}\]
done
clear
View Answer play_arrow
question_answer 46) The value of Plancks constant is \[\text{6}\text{.63 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-34}}}\text{Js}\text{.}\] The speed of light is \[\text{3 }\!\!\times\!\!\text{ 1}{{\text{0}}^{17}}\text{nm}{{\text{s}}^{\text{-1}}}\text{.}\]Which value is closest to the wavelength in nanometer of a quantum of light with frequency of \[\text{6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{15}}{{\text{s}}^{\text{-1}}}?\]
A)
10
done
clear
B)
25
done
clear
C)
50
done
clear
D)
75
done
clear
View Answer play_arrow
question_answer 47) What is the maximum numbers of electrons that can be .associated with the following set of quantum numbers? \[n=3,l=1\] and m = - 1
A)
10
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 48) What is the activation energy for a reaction if its rate doubles when the temperature is raised from \[20{}^\circ C\] to \[34{}^\circ C\]? (\[\text{R=}\,\text{8}\text{.314}\,\text{J}\,\text{mo}{{\text{l}}^{\text{-1}}}\,{{\text{K}}^{\text{-1}}}\])
A)
\[\text{342}\,\text{kJ}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
B)
\[269\,\text{kJ}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[34.7\,\text{kJ}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
\[15.1\,\text{kJ}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
question_answer 49) A hydrogen gas electrode is made by dipping platinum wire in a solution of\[HCl\]of \[pH=10\] and by passing hydrogen gas around the platinum wire at 1 atm pressure. The oxidation potential of electrode would be
A)
0.059 V
done
clear
B)
0.59 V
done
clear
C)
0.118 V
done
clear
D)
1.18 V
done
clear
View Answer play_arrow
question_answer 50) A reaction having equal energies of activation for forward and reverse reactions has
A)
\[\Delta S=0\]
done
clear
B)
\[\Delta G=0\]
done
clear
C)
\[\Delta H=0\]
done
clear
D)
\[\Delta H=\Delta G=\Delta S=0\]
done
clear
View Answer play_arrow
question_answer 51) At \[25{}^\circ C\] molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 \[\text{oh}{{\text{m}}^{\text{-1}}}\text{c}{{\text{m}}^{\text{2}}}\]\[\text{mo}{{\text{l}}^{\text{-1}}}\]and at infinite dilution its molar conductance is \[\text{238}\,\text{oh}{{\text{m}}^{\text{-1}}}\]\[\text{c}{{\text{m}}^{2}}\]\[\text{mo}{{\text{l}}^{\text{-1}}}.\]The degree of ionization of ammonium hydroxide at the same concentration and temperature is
A)
2.080%
done
clear
B)
20.800%
done
clear
C)
4.008%
done
clear
D)
40.800%
done
clear
View Answer play_arrow
question_answer 52) Based on equation \[E=-2.178\times {{10}^{-18}}J\left( \frac{{{Z}^{2}}}{{{n}^{2}}} \right)\]certain con-elusions are written. Which of them is not correct?
A)
The negative sign in equation simply means that the energy of electron bound to the nucleus is lower than it would be if the electrons were at the infinite distance from the nucleus
done
clear
B)
Larger the value of n, the larger is the orbit radius
done
clear
C)
Equation can be used to calculate the change in energy when the electron changes orbit
done
clear
D)
For n = 1 the electron has a more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit
done
clear
View Answer play_arrow
question_answer 53)
A button cell used in watches functions as following \[Zn(s)+A{{g}_{2}}O(s)+{{H}_{2}}O(l)2Ag(s)\] \[+Z{{n}^{2+}}(aq)+2O{{H}^{-}}(aq)\]
If half-cell potentials are \[Z{{n}^{2+}}(aq)+2{{e}^{-}}\to Zn(s){{E}^{o}}=-0.76V\] \[A{{g}_{2}}O(s)+{{H}_{2}}O(l)+2{{e}^{-}}\] \[\to 2Ag(s)+2O{{H}^{-}}(aq),\]\[{{E}^{o}}=0.34V\]
The cell potential will be
A)
1.10 V
done
clear
B)
0, 42 V
done
clear
C)
0.84 V
done
clear
D)
1.34 V
done
clear
View Answer play_arrow
question_answer 54) How many grams of concentrated nitric acid solution should be used to prepare 250 mL of \[\text{2}\text{.0M}\]\[\text{HN}{{\text{O}}_{\text{3}}}\] The concentrated acid is 70% \[\text{HN}{{\text{O}}_{\text{3}}}\].
A)
45.0 g cone. \[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
B)
90.0 g conc.\[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
C)
70.0 g cone. \[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
D)
54.0 g conc.\[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 55) The number of carbon atoms per unit cell of diamond unit cell is
A)
4
done
clear
B)
8
done
clear
C)
6
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 56) Maximum deviation from ideal gas is expected from
A)
\[{{H}_{2}}(g)\]
done
clear
B)
\[{{N}_{2}}(g)\]
done
clear
C)
\[C{{H}_{2}}(g)\]
done
clear
D)
\[N{{H}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 57) A metal has a fee lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g \[c{{m}^{-3}}\]. The molar mass of the metal is (\[{{N}_{A}}\] Avogadros constant \[=6.02\times {{10}^{23}}mo{{l}^{-1}}\])
A)
\[\text{40}\,\text{g}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
B)
\[\text{30}\,\text{g}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[27\,\text{g}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
\[20\,\text{g}\,\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
question_answer 58) Dipole-induced dipole interactions are present in which of the following pairs?
A)
\[{{\text{H}}_{\text{2}}}\text{O}\] and alcohol
done
clear
B)
\[\text{C}{{\text{l}}_{\text{2}}}\] and \[\text{CC}{{\text{l}}_{4}}\]
done
clear
C)
\[\text{HCl}\] and He atoms
done
clear
D)
\[\text{Si}{{\text{F}}_{4}}\] and He atoms
done
clear
View Answer play_arrow
question_answer 59) A magnetic moment of 1.73 BM will be shown by one among the following
A)
\[{{\text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
B)
\[{{\text{ }\!\![\!\!\text{ (NiCN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\]
done
clear
C)
\[\text{TiC}{{\text{l}}_{\text{4}}}\]
done
clear
D)
\[{{\text{ }\!\![\!\!\text{ CoC}{{\text{l}}_{6}}]}^{4-}}\]
done
clear
View Answer play_arrow
question_answer 60) Roasting of sulphides gives the gas X as a by-product. This is a colour less gas with .choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic acts as a reducing agent and its acid has never been insolated. The gas X is
A)
\[{{\text{H}}_{\text{2}}}\text{S}\]
done
clear
B)
\[\text{S}{{\text{O}}_{\text{2}}}\]
done
clear
C)
\[\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
D)
\[\text{S}{{\text{O}}_{3}}\]
done
clear
View Answer play_arrow
question_answer 61) Which is the strongest acid in the following?
A)
\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{4}}\]
done
clear
B)
\[\text{HCl}{{\text{O}}_{3}}\]
done
clear
C)
\[\text{HCl}{{\text{O}}_{4}}\]
done
clear
D)
\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{3}}\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following is paramagnetic?
A)
CO
done
clear
B)
\[\text{O}_{\text{2}}^{\text{-}}\]
done
clear
C)
\[\text{C}{{\text{N}}^{\text{-}}}\]
done
clear
D)
\[\text{N}{{\text{O}}^{+}}\]
done
clear
View Answer play_arrow
question_answer 63) Which of the following structure is similar to graphite?
A)
BN
done
clear
B)
B
done
clear
C)
\[{{\text{B}}_{\text{4}}}\text{C}\]
done
clear
D)
\[{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
done
clear
View Answer play_arrow
question_answer 64) The basic structural unit of silicates is
A)
\[\text{Si}{{\text{O}}^{\text{-}}}\]
done
clear
B)
\[\text{SiO}_{4}^{4-}\]
done
clear
C)
\[\text{SiO}_{3}^{2-}\]
done
clear
D)
\[\text{SiO}_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 65) Reaction by which benzaldehyde cannot be prepared?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 66) Which of the following does not give oxygen on heating?
A)
\[\text{KCl}{{\text{O}}_{\text{3}}}\]
done
clear
B)
\[\text{Zn(Cl}{{\text{O}}_{\text{3}}}{{)}_{2}}\]
done
clear
C)
\[{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{7}}\]
done
clear
D)
\[{{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\]
done
clear
View Answer play_arrow
question_answer 67) Which of the following lanthanides ions is diamagnetic? (At. nos. Ce = 58, Sm = 62, Eu = 63, Yb=70)
A)
\[\text{C}{{\text{e}}^{\text{2+}}}\]
done
clear
B)
\[\text{S}{{\text{m}}^{\text{2+}}}\]
done
clear
C)
\[\text{E}{{\text{u}}^{\text{2+}}}\]
done
clear
D)
\[\text{Y}{{\text{b}}^{\text{2+}}}\]
done
clear
View Answer play_arrow
question_answer 68) Identify the correct order of solubility in aqueous medium.
A)
\[\text{CuS}\,\text{}\,\text{Zn}\,\text{}\,\text{N}{{\text{a}}_{\text{2}}}\text{S}\]
done
clear
B)
\[\text{ZnS}\,\text{}\,\text{N}{{\text{a}}_{\text{2}}}\text{S}\,\,\text{}\,\text{CuS}\]
done
clear
C)
\[\,\text{N}{{\text{a}}_{\text{2}}}\text{S}\,\,\text{}\,\text{CuS}\,\text{ZnS}\]
done
clear
D)
\[\,\text{N}{{\text{a}}_{\text{2}}}\text{S}\,\,\text{}\,\text{ZnS}\,\text{}\,\text{CuS}\]
done
clear
View Answer play_arrow
question_answer 69) \[\text{Xe}{{\text{F}}_{\text{2}}}\] is structural with
A)
\[\text{Te}{{\text{F}}_{\text{2}}}\]
done
clear
B)
\[lCl_{2}^{-}\]
done
clear
C)
\[SbC{{l}_{3}}\]
done
clear
D)
\[\text{BaC}{{\text{l}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 70) An excess of \[\text{AgN}{{\text{O}}_{3}}\] is added to 100 mL of a 0.01 M solution of Dichlorotetraaquachromium\[\text{(III)}\]chloride. The number of moles of \[\text{AgCl}\] precipitate would be
A)
0.001
done
clear
B)
0.002
done
clear
C)
0.003
done
clear
D)
0.01
done
clear
View Answer play_arrow
question_answer 71) Which of these is least likely to act as a Lewis base?
A)
CO
done
clear
B)
\[{{\text{F}}^{\text{-}}}\]
done
clear
C)
\[\text{B}{{\text{F}}_{\text{3}}}\]
done
clear
D)
\[\text{P}{{\text{F}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 72) \[\text{KMn}{{\text{O}}_{\text{4}}}\] can be prepared from \[{{\text{K}}_{\text{2}}}\text{Mn}{{\text{O}}_{\text{4}}}\] as per reaction\[\text{3MnO}_{4}^{2-}+2{{H}_{2}}O2MnO_{4}^{-}\]\[+Mn{{O}_{2}}+4O{{H}^{-}}\] The reaction can go to completion by removing \[\text{O}{{\text{H}}^{\text{-}}}\] ions by adding
A)
HCI
done
clear
B)
KOH
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 73) Which of the following is electron deficient?
A)
\[{{(C{{H}_{3}})}_{2}}\]
done
clear
B)
\[{{(Si{{H}_{3}})}_{2}}\]
done
clear
C)
\[{{(B{{H}_{3}})}_{2}}\]
done
clear
D)
\[P{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 74) Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy- 4-methylhex-3-en-5-ynoic acid is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 75) Which of these is not a monomer for a high molecular mass silicone polymer?
A)
\[\text{MeSiC}{{\text{l}}_{\text{3}}}\]
done
clear
B)
\[\text{M}{{\text{e}}_{\text{2}}}\text{SiC}{{\text{l}}_{2}}\]
done
clear
C)
\[\text{M}{{\text{e}}_{3}}\text{SiCl}\]
done
clear
D)
\[\text{PhSiC}{{\text{l}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following statements about the interstitial compounds is incorrect?
A)
They retain metallic conductivity
done
clear
B)
They are chemically reactive
done
clear
C)
They are much harder than the pure metal
done
clear
D)
They have higher melting points than the pure metal
done
clear
View Answer play_arrow
question_answer 77) Which one of the following molecules contain no \[\text{ }\!\!\pi\!\!\text{ -}\]bond?
A)
\[\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
B)
\[{{\text{H}}_{\text{2}}}\text{O}\]
done
clear
C)
\[\text{S}{{\text{O}}_{\text{2}}}\]
done
clear
D)
\[\text{N}{{\text{O}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 78) Antiseptics and disinfectants either kill or prevent growth of microorganisms. Identify which of the following is not true.
A)
A 0.2% solution of phenol is an antiseptic while 1% solution acts as a disinfectant
done
clear
B)
Chlorine and iodine are used as strong disinfectants
done
clear
C)
Dilute solutions of boric acid and hydrogen, peroxide are strong antiseptics
done
clear
D)
Disinfectants harm the living tissues
done
clear
View Answer play_arrow
question_answer 79) Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-O-C{{H}_{3}}\]
done
clear
C)
\[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,\text{-O-C}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\mathop{\text{CH}}}\,\text{-C}{{\text{H}}_{\text{2}}}\text{-O-C}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 80) Nylon is an example of
A)
polyster
done
clear
B)
polysaccharide
done
clear
C)
polyamide
done
clear
D)
polythene
done
clear
View Answer play_arrow
question_answer 81) The structure of isobutyl group in an organic compound is
A)
done
clear
B)
\[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\text{ }\!\!|\!\!\text{ }}{\mathop{\text{CH}}}\,\text{-C}{{\text{H}}_{\text{2}}}\text{-C}{{\text{H}}_{\text{3}}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,\text{-}\]
done
clear
View Answer play_arrow
question_answer 82) Nitrobenzene on reaction with cone. \[HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}\]at \[80-100{}^\circ C\] forms which one of the following products?
A)
1, 2-dinitrobenzene
done
clear
B)
1, 3-dinitrobenzene
done
clear
C)
1, 4-dinitrobenzene
done
clear
D)
1, 2, 4-trinitrobenzene
done
clear
View Answer play_arrow
question_answer 83) Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
A)
\[-\text{C}\equiv \text{N}\]
done
clear
B)
\[-\text{S}{{\text{O}}_{\text{3}}}\text{H}\]
done
clear
C)
\[-\text{COOH}\]
done
clear
D)
\[-\text{N}{{\text{O}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 84) \[6.02\times {{10}^{20}}\]molecules of urea are present in 100 mL of its solution. The concentration of solution is
A)
0.02 M
done
clear
B)
0.01 M
done
clear
C)
0.001 M
done
clear
D)
0.1 M
done
clear
View Answer play_arrow
question_answer 85) Which of the following is a polar molecule?
A)
\[\text{B}{{\text{F}}_{\text{3}}}\]
done
clear
B)
\[\text{S}{{\text{F}}_{\text{3}}}\]
done
clear
C)
\[\text{Si}{{\text{F}}_{4}}\]
done
clear
D)
\[\text{Xe}{{\text{F}}_{4}}\]
done
clear
View Answer play_arrow
question_answer 86) Which is the monomer of neoprene in the following?
A)
\[C{{H}_{2}}=CH-C\equiv CH\]
done
clear
B)
\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-CH=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,-CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 87)
In the reaction, A is
A)
\[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{2}}\]and\[{{H}_{2}}O\]
done
clear
D)
\[{{H}^{+}}/{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 88)
The radical
A)
6 p-orbitals and 6 unpaired electrons
done
clear
B)
7 p-orbitals and 6 unpaired electrons
done
clear
C)
7 p-orbitals and 7 unpaired electrons
done
clear
D)
6 p-orbitals and 7 unpaired electrons
done
clear
View Answer play_arrow
question_answer 89) The order of stability of the following tatutomeric compound is \[\underset{\text{I}}{\mathop{\text{CH}{{ }_{\text{2}}}\text{=}\overset{\begin{smallmatrix} \text{OH} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{-C}{{\text{H}}_{\text{2}}}\text{-CH}{{ }_{\text{2}}}-\overset{\begin{smallmatrix} \text{OH} \\ \text{ }\!\!|\!\!\text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{-C}{{\text{H}}_{3}}}}\,\]\[\underset{\text{II}}{\mathop{\text{CH}{{ }_{3}}\text{=}\overset{\begin{smallmatrix} \text{OH} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{-C}{{\text{H}}_{\text{2}}}-\overset{\begin{smallmatrix} \text{OH} \\ \text{ }\!\!|\!\!\text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{-C}{{\text{H}}_{3}}}}\,\]\[\underset{\text{III}}{\mathop{\text{C}{{\text{H}}_{\text{3}}}\text{-}\overset{\begin{smallmatrix} \text{OH} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{=CH-}\overset{\begin{smallmatrix} \text{O} \\ \text{ }\!\!|\!\!\text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{-C}{{\text{H}}_{\text{3}}}}}\,\]
A)
\[I>II>III\]
done
clear
B)
\[III>II>I\]
done
clear
C)
\[II>I>III\]
done
clear
D)
\[II>III>I\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following compounds will not undergo Friedal-Crafts reaction easily?
A)
Cumene
done
clear
B)
Xylene
done
clear
C)
Nitrobenzene
done
clear
D)
Toluene
done
clear
View Answer play_arrow
question_answer 91) Select the wrong statement.
A)
Isogametes are similar in structure, function and behaviour
done
clear
B)
An isogametes differ either in structure, function and behaviour
done
clear
C)
In oomycetes female gamete is smaller and motile, while male gamete is larger and non-motile
done
clear
D)
Chlamydomonas exhibits both isogamy and anisogamy and Fucus shows oogamy
done
clear
View Answer play_arrow
question_answer 92) Which one of the following is not a correct statement?
A)
Herbarium houses dried, pressed and preserved plant specimens
done
clear
B)
Botanical gardens have collection of living plants for reference
done
clear
C)
A museum has collection of photographs of plants and animals
done
clear
D)
Key is a taxonomic aid for identification of Specimens
done
clear
View Answer play_arrow
question_answer 93) Isogamous condition with non-flagellated gametes is found in
A)
Chlamydomonas
done
clear
B)
Spirogyra
done
clear
C)
Volvox
done
clear
D)
Fucus
done
clear
View Answer play_arrow
question_answer 94) Besides paddy fields, cyan bacteria are also found inside vegetative part of
A)
Pinus
done
clear
B)
Cycas
done
clear
C)
Equisetum
done
clear
D)
Psilotum
done
clear
View Answer play_arrow
question_answer 95) Megasporangium is equivalent to
A)
embryo sac
done
clear
B)
fruit
done
clear
C)
nucellus
done
clear
D)
ovule
done
clear
View Answer play_arrow
question_answer 96)
Read the following statements (IV) and answer the question which follows them I. In liverworts, mosses and ferns gametophytes are free living. II. Gymnosperms and some ferns are heterospores. III. Sexual reproduction in Fucus, Volvox and Albugo is oogamous. IV. The saprophyte in liverworts is more elaborate than that in mosses.
How many of the above statements are correct?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 97) Among bitter gourd, mustard, brinjal, pumpkin, China rose, lupin, cucumber, sunnhemp, gram, guava, bean, chilli, plum, Petunia, tomato, rose, Withania, potato, onion, aloe and tulip, how many plants have hypogynous flower?
A)
Six
done
clear
B)
Ten
done
clear
C)
Fifteen
done
clear
D)
Eighteen
done
clear
View Answer play_arrow
question_answer 98) Interfascicular cambium develops from the cells of
A)
medullary rays
done
clear
B)
xylem parenchyma
done
clear
C)
endodermis
done
clear
D)
pericycle
done
clear
View Answer play_arrow
question_answer 99) In China rose the flowers are
A)
actinomorphic, hypogynous with twisted aestivation
done
clear
B)
actinomorphic, epigynous with valvate aestivation
done
clear
C)
zygonnorphic, hypogynous with imbricate aestivation
done
clear
D)
zygomorphic, epigynous with twisted aestivation
done
clear
View Answer play_arrow
question_answer 100) Lenticels are involved in
A)
transpiration
done
clear
B)
gaseous exchange
done
clear
C)
food transport
done
clear
D)
photosynthesis
done
clear
View Answer play_arrow
question_answer 101) Age of a tree can be estimated by
A)
its height and girth
done
clear
B)
biomass
done
clear
C)
number of annual rings
done
clear
D)
diameter of its heartwood
done
clear
View Answer play_arrow
question_answer 102) Seed coat is not thin, membranous in
A)
maize
done
clear
B)
coconut
done
clear
C)
groundnut
done
clear
D)
gram
done
clear
View Answer play_arrow
question_answer 103) Transition state structure of the substrate formed during an enzymatic reaction is
A)
transient but stable
done
clear
B)
permanent but unstable
done
clear
C)
transient and unstable
done
clear
D)
permanent and stable
done
clear
View Answer play_arrow
question_answer 104) A phosphoglyceride is always made up of
A)
only a saturated fatty acid esterifies to a glycerol molecule to which a phosphate group is also attached
done
clear
B)
only an unsaturated fatty acid esterifies to a glycerol molecule to which a phosphate group is also attached
done
clear
C)
a saturated or unsaturated fatty acid esterifies to a glycerol molecule to which a phosphate group is also attached
done
clear
D)
a saturated or unsaturated fatty acid esterifies to a phosphate group, which is also attached to a glycerol molecule
done
clear
View Answer play_arrow
question_answer 105) Pigment-containing membranous extensions in some cyan bacteria are
A)
heterocyst
done
clear
B)
basal bodies
done
clear
C)
pneumatophores
done
clear
D)
chromatophores
done
clear
View Answer play_arrow
question_answer 106) A major site for synthesis of lipids is
A)
RER
done
clear
B)
SER
done
clear
C)
symplast
done
clear
D)
nucleoplasm
done
clear
View Answer play_arrow
question_answer 107) The complex formed by a pair of synapsed homologous chromosomes is called
A)
equatorial plate
done
clear
B)
kinetochore
done
clear
C)
bivalent
done
clear
D)
axon me
done
clear
View Answer play_arrow
question_answer 108)
The three boxes in this diagram represent the three major biosynthetic pathways in aerobic respiration.
A)
NADH
done
clear
B)
ATP
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[FA{{D}^{+}}orFAD{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 109) The most abundant intracellular cation is
A)
\[N{{a}^{+}}\]
done
clear
B)
\[C{{a}^{2+}}\]
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 110) During seed germination its stored food is mobilized by
A)
ethylene
done
clear
B)
cytokinin
done
clear
C)
ABA
done
clear
D)
gibberellin
done
clear
View Answer play_arrow
question_answer 111) Which of the following criteria does not pertain to facilitated transport?
A)
Requirement of special membrane proteins
done
clear
B)
High selectivity
done
clear
C)
Transport saturation
done
clear
D)
Uphill transport
done
clear
View Answer play_arrow
question_answer 112) The first stable product of fixation of atmospheric nitrogen in leguminous plants is
A)
\[NO_{2}^{-}\]
done
clear
B)
ammonia
done
clear
C)
\[NO_{3}^{-}\]
done
clear
D)
glutamate
done
clear
View Answer play_arrow
question_answer 113) Which of the metabolites is common to respiration mediated breakdown of fats, carbohydrates and proteins?
A)
Glucose-6-phosphate
done
clear
B)
Fructose 1, 6-bisphosphate
done
clear
C)
Pyruvic acid
done
clear
D)
Acetyl Co-A
done
clear
View Answer play_arrow
question_answer 114) Which one of the following statement is correct?
A)
Hard outer layer of pollen is called intone
done
clear
B)
Sporogenous tissue is haploid
done
clear
C)
Endothelium produces the microspores
done
clear
D)
Tarentum nourishes the developing pollen
done
clear
View Answer play_arrow
question_answer 115) Product of sexual reproduction generally generates
A)
longer viability of seeds
done
clear
B)
prolonged dormancy
done
clear
C)
new genetic combination leading to variation
done
clear
D)
large biomass
done
clear
View Answer play_arrow
question_answer 116) Meiosis takes place in
A)
Meiocyte
done
clear
B)
Conidia
done
clear
C)
Gemmule
done
clear
D)
Megaspore
done
clear
View Answer play_arrow
question_answer 117) Advantage of cleistogamy is
A)
higher genetic variability
done
clear
B)
more vigorous offspring
done
clear
C)
no dependence on pollinators
done
clear
D)
vivipary
done
clear
View Answer play_arrow
question_answer 118) Monoecious plant of Chara shows occurrence of
A)
antheridiophore and archegoniophore on the same plant
done
clear
B)
stamen and carpel on the same plant
done
clear
C)
upper antheridium and lower oogonium on the same plant
done
clear
D)
upper oogonium and lower antheridium on the same plant
done
clear
View Answer play_arrow
question_answer 119) Perisperm differs from endosperm in
A)
being a haploid tissue
done
clear
B)
having no reserve food
done
clear
C)
being a diploid tissue
done
clear
D)
its formation by fusion of secondary nucleus with several sperms
done
clear
View Answer play_arrow
question_answer 120) Which of the following statements is not true of two genes that show 50% recombination frequency?
A)
the genes may be on different chromosomes
done
clear
B)
the genes are tightly linked
done
clear
C)
the genes show independent assortment
done
clear
D)
if the genes are present on the same chromosome, they undergo more than one crossovers in every meiosis
done
clear
View Answer play_arrow
question_answer 121) Variation in gene frequencies within populations can occur by chance rather than by natural selection. This is referred to as
A)
genetic flow
done
clear
B)
genetic drift
done
clear
C)
random mating
done
clear
D)
genetic load
done
clear
View Answer play_arrow
question_answer 122) If two persons with AB' blood group marry and have sufficiently large number of children, these children could be classified as A' blood group : AB' blood group : 'B' blood group in 1 : 2 : 1 ratio. Modern technique of protein electrophoresis reveals presence of both A' and 'B' type proteins in AB' blood group individuals. This is an example of
A)
co dominance
done
clear
B)
incomplete dominance
done
clear
C)
partial dominance
done
clear
D)
complete dominance
done
clear
View Answer play_arrow
question_answer 123) The process by which organisms with different evolutionary history evolve similar phenotypic adaptations in response to a common environmental challenge, is called
A)
natural selection
done
clear
B)
convergent evolution
done
clear
C)
non-random evolution
done
clear
D)
adaptive radiation
done
clear
View Answer play_arrow
question_answer 124) The tendency of population to remain in genetic equilibrium may be disturbed by
A)
random mating
done
clear
B)
lack of migration
done
clear
C)
lack of mutations
done
clear
D)
lack of random mating
done
clear
View Answer play_arrow
question_answer 125) Which of the following Bt crops is being grown in India by the farmers?
A)
Maize
done
clear
B)
Cotton
done
clear
C)
Brinjal
done
clear
D)
Soyabean
done
clear
View Answer play_arrow
question_answer 126) A good producer of citric acid is
A)
Aspergillums
done
clear
B)
Pseudomonas
done
clear
C)
Clostridium
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 127) DNA fragments generated by the restriction endonucleases in a chemical reaction can be separated by
A)
centrifugation
done
clear
B)
polymerase chain reaction
done
clear
C)
electrophoresis
done
clear
D)
restriction mapping
done
clear
View Answer play_arrow
question_answer 128) Which of the following is not correctly matched for the organism and its cell wall degrading enzyme?
A)
Bacteria - Lysozyme
done
clear
B)
Plant cells - Cellulase
done
clear
C)
Algae - Methylase
done
clear
D)
Fungi - Chitinase
done
clear
View Answer play_arrow
question_answer 129) The colonies of recombinant bacteria appear white in contrast to blue colonies of non-recombinant bacteria because of
A)
Non-recombinant bacteria containing \[\text{ }\!\!\beta\!\!\text{ -}\]galactosidase
done
clear
B)
Inspectional inactivation of \[\alpha \text{-}\]galactosidasein non-recombinant bacteria
done
clear
C)
Insertional inactivation of \[\alpha \text{-}\]galactosidase in recombinant bacteria
done
clear
D)
Inactivation of glycosidase enzyme in recombinant bacteria
done
clear
View Answer play_arrow
question_answer 130) Which of the following are likely to be present in deep sea water?
A)
Archaebacteria
done
clear
B)
Eubacteria
done
clear
C)
Blue-green algae
done
clear
D)
Saprophytic fungi
done
clear
View Answer play_arrow
question_answer 131) Natural reservoir of phosphorus is
A)
sea water
done
clear
B)
animal bones
done
clear
C)
rock
done
clear
D)
fossils
done
clear
View Answer play_arrow
question_answer 132) Secondary productivity is rate of formation of new organic matter by
A)
producer
done
clear
B)
parasite
done
clear
C)
consumer
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 133) Which one of the following is not used for ex situ plant conservation?
A)
Field gene banks
done
clear
B)
Seed banks
done
clear
C)
Shifting cultivation
done
clear
D)
Botanical gardens
done
clear
View Answer play_arrow
question_answer 134) Kyoto Protocol was endorsed at
A)
CoP-3
done
clear
B)
CoP-5
done
clear
C)
CoP-6
done
clear
D)
CoP-4
done
clear
View Answer play_arrow
question_answer 135) Which of the following represent maximum number of species among global biodiversity?
A)
Algae
done
clear
B)
Lichens
done
clear
C)
Fungi
done
clear
D)
Mosses and ferns
done
clear
View Answer play_arrow
question_answer 136) Match the name of the animal (Column I) with one characteristics (Column II) and the phylum/class (Column III) to which it belongs
A)
Column I - Petromyzon, Column II - Ectoparasite, Column III - Cyclostomata
done
clear
B)
Column I - Ichthyophis, Column II - Terrestrial, Column III - Reptilia
done
clear
C)
Column I - Limulus, Column II - Body covered by chitinous exoskeleton , Column III - Pisces
done
clear
D)
Column I - Adamsia, Column II - Radially symmetrical , Column III - Porifera
done
clear
View Answer play_arrow
question_answer 137) Which of the following are correctly matched with respect to their taxonomic classification?
A)
Flying fish, cuttlefish, silverfish - Pisces
done
clear
B)
Centipede, millipede, spider, scorpion -Insecta
done
clear
C)
House fly, butterfly, tse-tse fly, silverfish- Insecta
done
clear
D)
Spiny anteater, sea urchin, sea cucumber Echinodermata
done
clear
View Answer play_arrow
question_answer 138) Which group of animals belong to the same phylum?
A)
Malarial parasite, Amoeba, mosquito
done
clear
B)
Earthworm, pinworm, tapeworm
done
clear
C)
Prawn, scorpion, Locusta
done
clear
D)
Sponge, sea anemone, starfish
done
clear
View Answer play_arrow
question_answer 139) One of the representative of Phylum-Arthropoda is
A)
cuttlefish
done
clear
B)
silverfish
done
clear
C)
pufferfish
done
clear
D)
flying fish
done
clear
View Answer play_arrow
question_answer 140) The H-zone in the skeletal muscle fibre is due to
A)
the absence of myofibrils in the central portion of A-band
done
clear
B)
the central gap between myosin filaments in the A-band
done
clear
C)
the central gap between actin filaments extending through myosin filaments in the A-band
done
clear
D)
extension of myosin filaments in the central portion of the A-band
done
clear
View Answer play_arrow
question_answer 141) What external changes are visible after the last moult of a cockroach nymph?
A)
Mandibles become harder
done
clear
B)
Anal cerci develop
done
clear
C)
Both fore wings and hind wings develop
done
clear
D)
Labium develops
done
clear
View Answer play_arrow
question_answer 142) The Golgi complex plays a major role
A)
in trapping the light and transforming it into chemical energy
done
clear
B)
in digesting proteins and carbohydrates
done
clear
C)
as energy transferring organelles
done
clear
D)
in post translational modification of proteins and glycosidation of lipids
done
clear
View Answer play_arrow
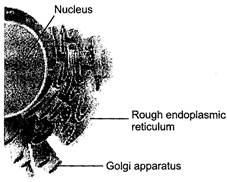
question_answer 143)
Which one of the following organelle in the figure correctly matches with its function?
A)
Rough endoplasmic reticulum, formation of glycoprotein?s
done
clear
B)
Golgi apparatus, protein synthesis
done
clear
C)
Golgi apparatus, formation of glycolipids
done
clear
D)
Rough endoplasmic reticulum, protein synthesis
done
clear
View Answer play_arrow
question_answer 144) Macromolecule chitin is
A)
nitrogen containing polysaccharide
done
clear
B)
phosphorus containing polysaccharide
done
clear
C)
sulphur containing polysaccharide
done
clear
D)
simple polysaccharide
done
clear
View Answer play_arrow
question_answer 145) The essential chemical components of many coenzymes are
A)
proteins
done
clear
B)
nucleic acids
done
clear
C)
carbohydrates
done
clear
D)
vitamins
done
clear
View Answer play_arrow
question_answer 146)
A stage in cell division is shown in the figure. Select the answer which gives correct identification of the stage with its characteristics
A)
Telophase - Nuclear envelope reforms, Golgi complex reforms
done
clear
B)
Late - Chromosomes move anaphase away from equatorial plate, Golgi complex not present
done
clear
C)
Cytokinesis - Cell plate formed, mitochondria distributed between two daughter cells
done
clear
D)
Telophase - Endoplasmic reticulum and nucleolus not reformed yet
done
clear
View Answer play_arrow
question_answer 147) Select the correct match of the digested products in humans given in column I with their absorption site and mechanism in column II.
A)
Column I - Glycine and glucose, Column II - Small intestine and active absorption
done
clear
B)
Column I - Fructose and \[N{{a}^{+}}\], Column II - Small intestine passive absorption
done
clear
C)
Column I - Glycerol and fatty acids, Column II - Duodenum and move as chi omicrons
done
clear
D)
Column I - Cholesterol and maltose, Column II - Large intestine and active absorption
done
clear
View Answer play_arrow
question_answer 148) A pregnant female delivers a baby, who suffers from stunted growth, mental retardation low intelligence quotient and abnormal skin. This is the result of
A)
deficiency of iodine in diet
done
clear
B)
low secretion of growth hormone
done
clear
C)
cancer of the thyroid gland
done
clear
D)
over secretion of pars distalis
done
clear
View Answer play_arrow
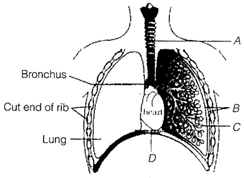
question_answer 149)
The figure shows a diagrammatic view of human respiratory system with labels A, B, C and D. Select the option, which gives correct identification and main function and/or characteristic.
A)
A-trachea-long tube supported by complete cartilaginous rings for conducting inspired air
done
clear
B)
B-pleural membrane-surround ribs on both sides to provide cushion against rubbing
done
clear
C)
C-alveoli-thin walled vascular bag-like structures for exchange of gases
done
clear
D)
D-lower end of lungs-diaphragm pulls it down during inspiration
done
clear
View Answer play_arrow
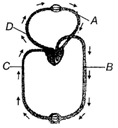
question_answer 150)
Figure shows schematic plan of blood circulation in human with labels A to D. Identify the label and give its function/s
A)
A-pulmonary vein-takes impure blood from body-parts, \[p{{O}_{2}}=60\,mmHg\]
done
clear
B)
B-pulmonary artery-takes blood from heart to lungs, \[p{{O}_{2}}=90\,mmHg\]
done
clear
C)
C-vena cava-takes blood from body parts to right auricle, \[pC{{O}_{2}}=45\,mmHg\]
done
clear
D)
D-dorsal aorta-takes blood from heart to body parts, \[p{{O}_{2}}=95\,mmHg\]
done
clear
View Answer play_arrow
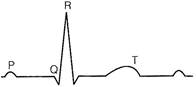
question_answer 151)
The diagram given here is the standard EGG of a normal person. The P-wave represents the
A)
contraction of both the atria
done
clear
B)
initiation of the ventricular contraction
done
clear
C)
beginning of the systole
done
clear
D)
end of systole
done
clear
View Answer play_arrow
question_answer 152)
Figure shows human urinary system with structures labelled A to D. Select option, which correctly identifies them and gives their characteristics and/of functions
A)
A-adrenal gland-located at the anterior part of kidney. Secrete catecholamines, which stimulate glycogen breakdown
done
clear
B)
B-pelvis-broad funnel shaped space inner to hilum, directly connected to loops of Henie
done
clear
C)
C-mediulla-inner zone of kidney and contains complete nephrons
done
clear
D)
D-cortex-outer part of kidney and do not contain any part of nephrons
done
clear
View Answer play_arrow
question_answer 153) Select the correct statement with respect to locomotion in humans
A)
A decreased level of progesterone causes osteoporosis in old people
done
clear
B)
Accumulation of uric acid crystals in joints causes their inflammation
done
clear
C)
The vertebral column has 10 thoracic vertebrae
done
clear
D)
The joint between adjacent vertebrae is a fibrous joint
done
clear
View Answer play_arrow
question_answer 154) The characteristics and an example of a synovial joint in humans is
A)
Characteristics - Fluid cartilage between two bones, limited movements, Examples - Knee joints
done
clear
B)
Characteristics - Fluid filled between two joints, provides cushion, Examples - Skull bones
done
clear
C)
Characteristics - Fluid filled synovial cavity between two bones, Examples - Joint between atlas and axis
done
clear
D)
Characteristics - Lymph filled between two bones, limited movement, Examples - Gliding joint between carpals
done
clear
View Answer play_arrow
question_answer 155)
A diagram showing axon terminal and synapse is given. Identify correctly at least two of A-D
A)
A-Receptor C-Synaptic vesicles
done
clear
B)
B-Synaptic connection D-K+
done
clear
C)
A-Neurotransmitter S-Synaptic cleft
done
clear
D)
C-Neurotransmitter D-Ca2+
done
clear
View Answer play_arrow
question_answer 156)
Parts A, B, C and D of the human eyes are shown in the diagram. Select the option, which gives correct identification along with its functions/characteristics
A)
A-retina-contains photoreceptors-rods and cones
done
clear
B)
B-blind spot-has only a few rods and cones
done
clear
C)
C-aqueous chamber-reflects the light, which does not pass through the lens
done
clear
D)
D-choroidits anterior part forms ciliary body
done
clear
View Answer play_arrow
question_answer 157) Which of the following statement is correct in relation to the endocrine system?
A)
Adenohypophysis is under direct neural regulation of the hypothalamus
done
clear
B)
Organs in the body like gastrointestinal tract, heart, kidney and liver do not produce any hormones
done
clear
C)
Non-nutrient chemicals produced by the body in trace amount that act as intercellular messenger are known as hormones
done
clear
D)
Releasing and inhibitory hormones are produced by the pituitary gland
done
clear
View Answer play_arrow
question_answer 158) Select the answer which correctly matches the endocrine gland with the hormone it secretes and its function/deficiency symptom
A)
Endocrine gland Hormone Function/deficiency symptoms Anterior pituitary Oxytocin Stimulates uterus contraction during child birth
done
clear
B)
Endocrine gland Hormone Function/deficiency symptoms Posterior pituitary Growth Hormone (GH) Over secretion stimulates abnormal growth
done
clear
C)
Endocrine gland Hormone Function/deficiency symptoms Thyroid gland Thyroxine Lack of iodine in diet results in goiter
done
clear
D)
Endocrine gland Hormone Function/deficiency symptoms Corpus Iuteum Testosterone Stimulates spermatogenesis
done
clear
View Answer play_arrow
question_answer 159) What is the correct sequence of sperm formation?
A)
Spermatid, Spermatocyte, Spermatogonia, Spermatozoa
done
clear
B)
Spermatogonia, Spermatocyte, Spermatozoa, Spermatid
done
clear
C)
Spermatogonia, Spermatozoa, Spermatocyte, Spermatid
done
clear
D)
Spermatogonia, Spermatocyte, Spermatid, Spermatozoa
done
clear
View Answer play_arrow
question_answer 160) Menstrual flow occurs due to lack of
A)
progesterone
done
clear
B)
FSH
done
clear
C)
oxytocin
done
clear
D)
vasopressin
done
clear
View Answer play_arrow
question_answer 161) Which one of the following is not the function of placenta? It
A)
facilitates supply of oxygen and nutrients to embryo
done
clear
B)
secretes estrogen
done
clear
C)
facilitates removal of carbon dioxide and waste material from embryo
done
clear
D)
secretes oxytocin during parturition
done
clear
View Answer play_arrow
question_answer 162) One of the legal methods of birth control is
A)
abortion by taking an appropriate medicine
done
clear
B)
by abstaining from coitus from day 10-17 of the menstrual cycle
done
clear
C)
by having coitus at the time of day break
done
clear
D)
by a premature ejaculation during coitus
done
clear
View Answer play_arrow
question_answer 163) Which of the following cannot be detected in a developing foetus by amniocentesis?
A)
Klinefelter's syndrome
done
clear
B)
Sex of the foetus
done
clear
C)
Down's syndrome
done
clear
D)
Jaundice
done
clear
View Answer play_arrow
question_answer 164) Artificial insemination means
A)
transfer of sperms of a healthy donor to a test-tube containing ova
done
clear
B)
transfer of sperms of husband to a test-tube containing ova
done
clear
C)
artificial introduction of sperms of a healthy donor into the vagina
done
clear
D)
introduction of sperms of healthy donor directly into the ovary
done
clear
View Answer play_arrow
question_answer 165) Which Mendelian idea is depicted by a cross in which the \[{{\text{F}}_{\text{1}}}\] generation resembles both the parents?
A)
Incomplete dominance
done
clear
B)
Law of dominance
done
clear
C)
Inheritance of one gene
done
clear
D)
Co dominance
done
clear
View Answer play_arrow
question_answer 166) The incorrect statement with regard to hemophilia is
A)
it is a sex-linked disease
done
clear
B)
it is a recessive disease
done
clear
C)
it is a dominant disease
done
clear
D)
a single protein involved in the clotting of blood is affected
done
clear
View Answer play_arrow
question_answer 167) If both parents are carriers for thalassemia, which is an autosomal recessive disorder, what are the chances of pregnancy resulting in an affected child?
A)
No chance
done
clear
B)
50%
done
clear
C)
25%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 168)
The diagram shows an important concept in the genetic implication of DNA. Fill in the blanks A to C
A)
A-transcription, B-replication, C-James Watson
done
clear
B)
A-translation, B-transcription, C-Erevin Chargaff
done
clear
C)
A-transcription, ^-translation, C-Francis Crick
done
clear
D)
A-translation, 6-extension, C-Rosalind Franklin
done
clear
View Answer play_arrow
question_answer 169) Which enzyme/s will be produced in a cell in which there is a non-sense mutation in the lac Y gene?
A)
\[\text{ }\!\!\beta\!\!\text{ -}\]galactosidase
done
clear
B)
Lactose permease
done
clear
C)
Transacetylase
done
clear
D)
Lactose permease and transacetylase
done
clear
View Answer play_arrow
question_answer 170) According to Darwin, the organic evolution is due to
A)
intraspecific competition
done
clear
B)
interspecific competition
done
clear
C)
competition within closely related species
done
clear
D)
reduced feeding efficiency in one species due to the presence of interfering species
done
clear
View Answer play_arrow
question_answer 171) The eyes of Octopus and eyes of cat show different patterns of structure, yet they perform similar function. This is an example of
A)
homologous organs that have evolved due to convergent evolution
done
clear
B)
homologous organs that have evolved due to divergent evolution
done
clear
C)
analogous organs that have evolved due to convergent evolution
done
clear
D)
analogous organs that have evolved due to divergent evolution
done
clear
View Answer play_arrow
question_answer 172) Infection of Ascaris usually occurs by
A)
drinking water containing egg of Ascaris
done
clear
B)
eating imperfectly cooked port
done
clear
C)
tse-tse fly
done
clear
D)
mosquito bite
done
clear
View Answer play_arrow
question_answer 173) The cell-mediated immunity inside the human body is carried out by
A)
T-lymphocytes
done
clear
B)
B-lymphocytes
done
clear
C)
thrombocytes
done
clear
D)
erythrocytes
done
clear
View Answer play_arrow
question_answer 174) In plant breeding programmers, the entire collection (of plants/seeds) having all the diverse alleles for all genes in a given crop is called
A)
selection of superior recombinants
done
clear
B)
cross-hybridisation among the selected parents
done
clear
C)
evaluation and selection of parents
done
clear
D)
germplasm collection
done
clear
View Answer play_arrow
question_answer 175) During sewage treatment, biogases are produced, which include
A)
methane, hydrogen sulphide and carbon dioxide
done
clear
B)
methane, oxygen and hydrogen sulphide
done
clear
C)
hydrogen sulphide, methane and sulphur dioxide
done
clear
D)
hydrogen sulphide, nitrogen and methane
done
clear
View Answer play_arrow
question_answer 176) A biologist studied the population of rats in a barn. He found that the average nasality was 250, average mortality 240, immigration 20 and emigration 30. The net increase in population is
A)
10
done
clear
B)
15
done
clear
C)
05
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 177) Which one of the following processes during decomposition is correctly described?
A)
Fragmentation-Carried out by organisms such as earthworm
done
clear
B)
Humiliation-Leads to the accumulation of a dark coloured substance humus, which undergoes microbial action at a very fast rate
done
clear
C)
Catabolism-Last step in the decomposition under fully anaerobic condition
done
clear
D)
Leaching-Water soluble inorganic nutrients rise to the top layers of soil
done
clear
View Answer play_arrow
question_answer 178) A sedentary sea anemone gets attached to the shell lining of hermit crab. The association is
A)
ectoparasitism
done
clear
B)
symbiosis
done
clear
C)
commensalism
done
clear
D)
amensalism
done
clear
View Answer play_arrow
question_answer 179) Global warming can be controlled by reducing deforestation, cutting down use of fossil fuel
A)
Reducing deforestation, cutting down use of fossil fuel
done
clear
B)
Reducing reforestation, increasing the use of fossil fuel
done
clear
C)
Increasing deforestation, slowing down the growth of human population
done
clear
D)
Increasing deforestation, reducing efficiency of energy usage
done
clear
View Answer play_arrow
question_answer 180) The Air Prevention and Control of Pollution Act came into force in
A)
1975
done
clear
B)
1981
done
clear
C)
1985
done
clear
D)
1990
done
clear
View Answer play_arrow
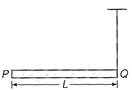
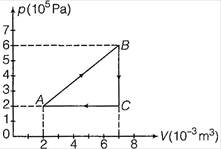
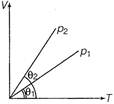
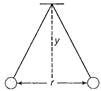
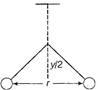
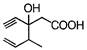
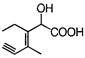
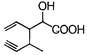
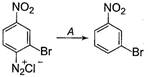
 Arrows numbered 4, 8 and 12 can all be
Arrows numbered 4, 8 and 12 can all be