question_answer 1) A tuning fork produce 2 beats when B sounded with one oscillator of frequency k 514 Hz, and produces 6 beats with the f other oscillator of frequency 510 Hz. The frequency of tuning fork is:
A)
516 Hz
done
clear
B)
510 Hz
done
clear
C)
514 Hz
done
clear
D)
520 Hz
done
clear
View Answer play_arrow
question_answer 2) Wavelength of the particle whose momentum is \[p:\]
A)
\[h/p\]
done
clear
B)
\[hp\]
done
clear
C)
\[p/h\]
done
clear
D)
\[p+h\]
done
clear
View Answer play_arrow
question_answer 3) Colour of a star indicates its:
A)
density
done
clear
B)
distance
done
clear
C)
energy
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 4) Which of the following has same dimensions to that of Planck's constant?
A)
Work
done
clear
B)
Energy
done
clear
C)
Linear momentum
done
clear
D)
Angular momentum
done
clear
View Answer play_arrow
question_answer 5) Temperature of a piece of silicon is raised from \[{{27}^{o}}C\] to \[{{100}^{o}}C,\] then its conductivity:
A)
increases
done
clear
B)
decreases
done
clear
C)
remains same
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 6) If a liquid does not wet a solid surface, then its angle of contact is:
A)
acute angle
done
clear
B)
obtuse angle
done
clear
C)
right angle
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 7) Three capacitors of 3, 2 and 6\[\mu F\] connected in series with a battery of 10V. Then charge on 3\[\mu F\] capacitor will be:
A)
\[10\mu C\]
done
clear
B)
\[12\mu C\]
done
clear
C)
\[14\mu C\]
done
clear
D)
\[5\mu C\]
done
clear
View Answer play_arrow
question_answer 8) To hear the echo in 1 second, the minimum L distance of the source from the reflecting surface should be:
A)
28 m
done
clear
B)
18 m
done
clear
C)
19 m
done
clear
D)
165 m
done
clear
View Answer play_arrow
question_answer 9) If length of a simple pendulum increases by 300%, then its time period increases by:
A)
100%
done
clear
B)
200%
done
clear
C)
300%
done
clear
D)
400%
done
clear
View Answer play_arrow
question_answer 10) Half life of radium is 1600 year, then its \[{{{\scriptstyle{}^{1}/{}_{16}}}^{th}}\] part will remain undecayed after:
A)
1200 year
done
clear
B)
6400 year
done
clear
C)
1800 year
done
clear
D)
2200 year
done
clear
View Answer play_arrow
question_answer 11) When a light beam enters into water from air then which of the following does not change?
A)
Velocity
done
clear
B)
Frequency
done
clear
C)
Wavelength
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 12) Sound waves produced in gas are:
A)
longitudinal
done
clear
B)
transversal
done
clear
C)
stationary
done
clear
D)
progressive
done
clear
View Answer play_arrow
question_answer 13) When a force F is applied on a wire of length L and radius r, extension produce is \[l\]. If same force F is applied on the same material wire of length 2L and radius 2r, then extension will be:
A)
\[l\]
done
clear
B)
\[2l\]
done
clear
C)
\[l/2\]
done
clear
D)
\[4l\]
done
clear
View Answer play_arrow
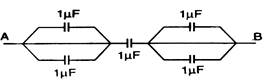
question_answer 14)
Equivalent capacity between A and B is:
A)
\[2\mu F\]
done
clear
B)
\[3\mu F\]
done
clear
C)
\[4\mu F\]
done
clear
D)
\[0.5\mu F\]
done
clear
View Answer play_arrow
question_answer 15) Angular velocity of second hand of a clock is:
A)
\[\frac{\pi }{30}rad/\sec \]
done
clear
B)
\[\frac{\pi }{60}rad/\sec \]
done
clear
C)
\[\frac{\pi }{15}rad/\sec \]
done
clear
D)
\[\frac{\pi }{2}rad/\sec \]
done
clear
View Answer play_arrow
question_answer 16) n-type semiconductor is:
A)
positively charged
done
clear
B)
neutral
done
clear
C)
negatively charged
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 17) Intensity of a source of a 100 candela at a distance 2 metre is:
A)
25 Lux
done
clear
B)
50 Lux
done
clear
C)
75 Lux
done
clear
D)
150 Lux
done
clear
View Answer play_arrow
question_answer 18) Atomic number of an atom is Z and mass number is m, then number of neutrons in its nucleus is:
A)
\[m-Z\]
done
clear
B)
\[\frac{m}{Z}\]
done
clear
C)
\[m+Z\]
done
clear
D)
\[m\times Z\]
done
clear
View Answer play_arrow
question_answer 19) Power factor of L-C-R circuit in resonation condition is:
A)
\[1\]
done
clear
B)
\[1/2\]
done
clear
C)
zero
done
clear
D)
\[1/\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 20) If tension of sonometer wire is made four times, then its frequency will change by a factor of:
A)
2
done
clear
B)
4
done
clear
C)
1/2
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 21) Which of the following has maximum specific heat?
A)
Water
done
clear
B)
Alcohol
done
clear
C)
Glycerene
done
clear
D)
Oil
done
clear
View Answer play_arrow
question_answer 22) Momentum of a body of mass 1 kg is 10 kg-m/s/ then its kinetic energy will be:
A)
\[\text{100}\,\text{J}\]
done
clear
B)
\[\text{50}\,\text{J}\]
done
clear
C)
\[\text{1000}\,\text{J}\]
done
clear
D)
\[\text{200}\,\text{J}\]
done
clear
View Answer play_arrow
question_answer 23) Nucleus of an atom has:
A)
\[e\,\text{and}\,n\]
done
clear
B)
\[e\,\text{and}\,p\]
done
clear
C)
\[p\,\text{and}\,n\]
done
clear
D)
\[e,p\,\text{and}\,n\]
done
clear
View Answer play_arrow
question_answer 24) Which of the following is scalar quantity?
A)
Current
done
clear
B)
Velocity
done
clear
C)
Force
done
clear
D)
Acceleration
done
clear
View Answer play_arrow
question_answer 25) Equation of a progressive sound wave is \[y=a\,\sin \left( 400\pi t-\frac{\pi x}{0.85} \right)\]where \[x\] in (metre), \[t\] (second)/ then frequency of wave is:
A)
200 Hz
done
clear
B)
400 Hz
done
clear
C)
500 Hz
done
clear
D)
600 Hz
done
clear
View Answer play_arrow
question_answer 26) Wavelength of the wave in the above question is:
A)
1.7m
done
clear
B)
8.5m
done
clear
C)
0.85 m
done
clear
D)
0.17 m
done
clear
View Answer play_arrow
question_answer 27) A wheel completes 2000 turns in completing a distance of 9.5 km, then diameter of the wheel is:
A)
1.5m
done
clear
B)
1.5 cm
done
clear
C)
7.5m
done
clear
D)
7.5 cm
done
clear
View Answer play_arrow
question_answer 28) In nth orbit of hydrogen atom energy \[e=\frac{13.6}{{{n}^{2}}}eV,\]then energy required to displace electrons from\[n=1\,\text{to}\,n=\,2\]is:
A)
-10.2 eV
done
clear
B)
3.4 eV
done
clear
C)
-13.6 eV
done
clear
D)
1.51 eV
done
clear
View Answer play_arrow
question_answer 29) Relation of wavelengths of sound an light is:
A)
\[{{\lambda }_{S}}<{{\lambda }_{L}}\]
done
clear
B)
\[{{\lambda }_{S}}>{{\lambda }_{L}}\]
done
clear
C)
\[{{\lambda }_{S}}={{\lambda }_{L}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 30) In Young's experiment, if separate between slits is made half, then fringe width will become:
A)
double
done
clear
B)
four times
done
clear
C)
half
done
clear
D)
remains same
done
clear
View Answer play_arrow
question_answer 31) Resolving power of human eye is:
A)
0.1
done
clear
B)
1
done
clear
C)
0.1?
done
clear
D)
1?
done
clear
View Answer play_arrow
question_answer 32) Dancing of small pieces of camphor on the surface of water is due to:
A)
viscosity
done
clear
B)
surface tension
done
clear
C)
weight
done
clear
D)
lifting force
done
clear
View Answer play_arrow
question_answer 33) Relation between specific heats of an ideal gas:
A)
\[Cp+{{C}_{\upsilon }}=R\]
done
clear
B)
\[{{C}_{P}}-{{C}_{\upsilon }}=R\]
done
clear
C)
\[\frac{{{C}_{P}}}{{{C}_{\upsilon }}}=R\]
done
clear
D)
\[\frac{{{C}_{\upsilon }}}{{{C}_{P}}}=R\]
done
clear
View Answer play_arrow
question_answer 34) Who gave the kinetic molecular theory of gas?
A)
Devic
done
clear
B)
Einestein
done
clear
C)
Newton
done
clear
D)
Bernoulli
done
clear
View Answer play_arrow
question_answer 35) Potential energy of a particle at a distance x from mean position, which is execute simple hormonic motion:
A)
\[\frac{1}{2}m{{\omega }^{2}}{{x}^{2}}\]
done
clear
B)
zero
done
clear
C)
\[{{m}^{2}}\]
done
clear
D)
\[\frac{1}{2}{{m}^{2}}{{\omega }^{2}}{{x}^{2}}\]
done
clear
View Answer play_arrow
question_answer 36) If distance between light source and screen is doubled, then intensity of light will become:
A)
half
done
clear
B)
double
done
clear
C)
remain unchanged
done
clear
D)
\[1/4\,th\]
done
clear
View Answer play_arrow
question_answer 37) The colour of a red cloth in green light appears:
A)
red
done
clear
B)
green
done
clear
C)
orange
done
clear
D)
black
done
clear
View Answer play_arrow
question_answer 38) An inductor and a capacitor are connected in an A.C. circuit inductance and capacitance are 1 H and 25 \[\mu F\] then for maximum current, angular frequency will be (circuit is connected in series):
A)
200 rad/sec
done
clear
B)
50 rad/sec
done
clear
C)
100 rad/sec
done
clear
D)
150 rad/sec
done
clear
View Answer play_arrow
question_answer 39) A satellite of mass m, revolving round a planet of mass M in a circular orbit of radius r, then orbital velocity of the satellite is:
A)
\[\frac{mg}{r}\]
done
clear
B)
\[\sqrt{\frac{GM}{r}}\]
done
clear
C)
\[\sqrt{\frac{2GM}{r}}\]
done
clear
D)
\[\frac{GM}{r}\]
done
clear
View Answer play_arrow
question_answer 40) Energy stored in the unit volume of a wire due to its elasticity is:
A)
\[\frac{1}{2}\] (force \[\times \] strain)
done
clear
B)
\[\frac{1}{2}\]stress \[\times \] strain
done
clear
C)
stress/strain
done
clear
D)
force/strain
done
clear
View Answer play_arrow
question_answer 41) Value of \[-40{}^\circ C\] in Fahrenheit scale is:
A)
\[-40{}^\circ F\]
done
clear
B)
\[32{}^\circ F\]
done
clear
C)
\[-32{}^\circ F\]
done
clear
D)
\[40{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 42) Spectrum of a star is shifting towards voilet colour, then the star:
A)
is going away from earth
done
clear
B)
is coming towards earth
done
clear
C)
intensity of light of star is increasing
done
clear
D)
intensity of light of star is decreasing
done
clear
View Answer play_arrow
question_answer 43) The amount of heat required to change \[1\,gm\,({{0}^{\text{o}}}C)\] of ice into water of \[{{100}^{o}}C,\] is:
A)
716 cal
done
clear
B)
500 cal
done
clear
C)
180 cal
done
clear
D)
100 cal
done
clear
View Answer play_arrow
question_answer 44) Susceptibility of a magnetic field is:
A)
\[\chi =\frac{l}{H}\]
done
clear
B)
\[\chi =\frac{B}{H}\]
done
clear
C)
\[\chi =\frac{M}{V}\]
done
clear
D)
\[\chi =\frac{M}{H}\]
done
clear
View Answer play_arrow
question_answer 45) Sphere, disc and ring are allowed to roll down on an inclined plane from its top, then order in which they reach at the bottom will be:
A)
ring, disc, sphere
done
clear
B)
sphere, disc, ring
done
clear
C)
disc, ring, sphere
done
clear
D)
sphere, ring, disc
done
clear
View Answer play_arrow
question_answer 46) If dopping in the P region is high then N region:
A)
depletion layer will more towards P.
done
clear
B)
depletion layer will more towards N.
done
clear
C)
depletion layer will remain unchanged.
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 47) Work of invertor is:
A)
to change A.C. into D.C.
done
clear
B)
to change D.C. to A.C.
done
clear
C)
to regulate voltage
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 48) Dimensions of torque is:
A)
\[[{{M}^{1}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{2}}{{L}^{2}}{{T}^{2}}]\]
done
clear
C)
\[[{{M}^{-1}}L{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{-2}}{{L}^{-2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 49) For a triode valve \[\mu \,=\,50,\,\Delta \,{{V}_{g}}\,=0.2\] volt then value of\[\Delta \,{{V}_{P}}\,\] is:
A)
5 volt
done
clear
B)
10 volt
done
clear
C)
0.2 volt
done
clear
D)
50 volt
done
clear
View Answer play_arrow
question_answer 50) Which of the following has maximum property of elasticity?
A)
Rubber
done
clear
B)
Lead
done
clear
C)
Wood
done
clear
D)
Steel
done
clear
View Answer play_arrow
question_answer 51) If we made half the radius of earth, then duration of day will become:
A)
6 hour
done
clear
B)
12 hour
done
clear
C)
24 hour
done
clear
D)
3 hour
done
clear
View Answer play_arrow
question_answer 52) A stone is dropped in 19.6 metre depth well, echo is heard in 2.06 second, then velocity of sound is:
A)
315.2m/s
done
clear
B)
320.5 m/s
done
clear
C)
326.7 m/s
done
clear
D)
332.4 m/s
done
clear
View Answer play_arrow
question_answer 53) An electron is moving with velocity\[\vec{v}\]in the direction of magnetic field \[\vec{B}\] then force acting on electron is :
A)
zero
done
clear
B)
\[e(\vec{\upsilon }\times \vec{B})\]
done
clear
C)
\[e(\vec{B}\times \vec{\upsilon })\]
done
clear
D)
200 Joule
done
clear
View Answer play_arrow
question_answer 54) If temperature of an object is \[140{}^\circ F\], then its temperature in centrigrade is:
A)
\[105{}^\circ C\]
done
clear
B)
\[32{}^\circ C\]
done
clear
C)
\[140{}^\circ C\]
done
clear
D)
\[60{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 55) Focal length of a lens is + 10 cm and its refractive index is 1.4, it is dipped in a liquid of refractive index 1.6, then its focal length becomes:
A)
- 16 cm
done
clear
B)
+ 32 cm
done
clear
C)
+ 16 cm
done
clear
D)
- 32 cm
done
clear
View Answer play_arrow
question_answer 56) Ten wires each of resistance 1 ohm are connected in parallel, then total resistance will be:
A)
10\[\Omega \]
done
clear
B)
1\[\Omega \]
done
clear
C)
0.1\[\Omega \]
done
clear
D)
0.0010\[\Omega \]
done
clear
View Answer play_arrow
question_answer 57) If focal length of convex lens is 9 cm and that of a concave lens is - 18 cm, then focal length of combination is:
A)
+ 9 cm
done
clear
B)
-18 cm
done
clear
C)
-9 cm
done
clear
D)
+18 cm
done
clear
View Answer play_arrow
question_answer 58) Two mirror are put at an angle of \[60{}^\circ \] then number of image of an object placed between the mirrors is:
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 59) An observer is coming towards plane mirror with a speed of 6 m/s, then apparent velocity of his image is :
A)
6 m/s
done
clear
B)
4 m/s
done
clear
C)
12 m/s
done
clear
D)
3 m/s
done
clear
View Answer play_arrow
question_answer 60) Speed of light in vacuum is.
A)
\[\frac{1}{\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
\[\sqrt{\frac{{{\mu }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
D)
\[\sqrt{\frac{{{\varepsilon }_{0}}}{{{\mu }_{0}}}}\]
done
clear
View Answer play_arrow
question_answer 61) In thermionic diode valve/ relation between, plate current and plate voltage is:
A)
\[{{I}_{P}}\propto {{V}_{P}}^{3/2}\]
done
clear
B)
\[{{I}_{P}}\propto {{V}_{P}}^{2/3}\]
done
clear
C)
\[{{I}_{P}}\propto {{V}_{P}}^{1/2}\]
done
clear
D)
\[{{I}_{P}}\propto {{V}_{P}}\]
done
clear
View Answer play_arrow
question_answer 62) In an A.C. circuit, for angular frequency \[\omega =\]1000 rad/s, resistance of a coil is 100 \[\Omega ,\] then for (\[\omega =\] 100 rad/s, resistance of the coil is:
A)
1000\[\Omega \]
done
clear
B)
100\[\Omega \]
done
clear
C)
50\[\Omega \]
done
clear
D)
10\[\Omega \]
done
clear
View Answer play_arrow
question_answer 63) In a secondary coil of a step up transformer:
A)
less turns of thin wire
done
clear
B)
more turns of thin wire
done
clear
C)
less turns of thick wire
done
clear
D)
more turns of thick wire
done
clear
View Answer play_arrow
question_answer 64) In photo electric effect intensity of incidence light is made double, then energy of emitted photo electrons will:
A)
increase
done
clear
B)
decrease
done
clear
C)
remain same
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 65) 1 eV is equivalent to:
A)
10 erg
done
clear
B)
\[1.1\times {{10}^{-11}}\,erg\]
done
clear
C)
\[1.6\times {{10}^{-38}}erg\]
done
clear
D)
\[1.6\,\times {{10}^{-19}}\,Joule\]
done
clear
View Answer play_arrow
question_answer 66) Unit of amplification factor of a triode is:
A)
volt
done
clear
B)
Ohm
done
clear
C)
Lyman
done
clear
D)
no unit
done
clear
View Answer play_arrow
question_answer 67) For a vacuum diode, slope of a characteristics curve is \[2\times {{10}^{-2}}\,mA{{V}^{-1}},\] then plate resistance of diode is:
A)
50 \[\Omega \]
done
clear
B)
50 \[\text{k}\Omega \]
done
clear
C)
500 \[\text{k}\Omega \]
done
clear
D)
500\[\Omega \]
done
clear
View Answer play_arrow
question_answer 68) In a copper wire 1.6 mA current is flowing, then number of free electrons moving in wire is :
A)
\[{{10}^{11}}\]
done
clear
B)
\[{{10}^{16}}\]
done
clear
C)
\[{{10}^{19}}\]
done
clear
D)
\[{{10}^{15}}\]
done
clear
View Answer play_arrow
question_answer 69) If p-n junction is unbiased then:
A)
current is zero, because drifting of charge is same from both sides
done
clear
B)
current is zero, because there is no movement of charge
done
clear
C)
current is not zero
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 70) Who found the charge on electron?
A)
Bohr
done
clear
B)
Milikan
done
clear
C)
Thomson
done
clear
D)
Rozen
done
clear
View Answer play_arrow
question_answer 71) If filament is heated, then it emits:
A)
proton
done
clear
B)
photon
done
clear
C)
electron
done
clear
D)
\[\alpha -\]particle
done
clear
View Answer play_arrow
question_answer 72) If wavelength of first line of Balmer series of an atom is \[6563\overset{\text{o}}{\mathop{\text{A}}}\,,\] then the wavelength of first line of Lyman series for same atom is:
A)
\[4848\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1216\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[909\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[776\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 73) Relation between half life (T) and mean life \[({{T}_{0}})\] for a radioactive element is:
A)
\[T=\frac{0.693}{{{T}_{o}}}\]
done
clear
B)
\[{{T}_{0}}=\frac{0.693}{T}\]
done
clear
C)
\[T=0.693\,{{T}_{0}}\]
done
clear
D)
\[{{T}_{0}}=0.693\,T\]
done
clear
View Answer play_arrow
question_answer 74) Half life of radioactive element is 30 days, After 90 days percentage decayed of the element will be :
A)
92.15%
done
clear
B)
87.5%
done
clear
C)
47%
done
clear
D)
18.5%
done
clear
View Answer play_arrow
question_answer 75) If pressure of water is increased, then it boils at a temperature ...\[100{}^\circ C\]:
A)
less than
done
clear
B)
more than
done
clear
C)
high temperature
done
clear
D)
will boil at critical temperature
done
clear
View Answer play_arrow
question_answer 76) Latent heat of 1 gm of steam is 536 cal/gm, then its value in Joule/kg is:
A)
\[2.25\,\times {{10}^{6}}\]
done
clear
B)
\[2.25\,\times {{10}^{3}}\]
done
clear
C)
2.25
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 77) Specific heat of ideal gas depends upon:
A)
\[T\]
done
clear
B)
\[1/T\]
done
clear
C)
\[\sqrt{T}\]
done
clear
D)
does not depend on its temperature
done
clear
View Answer play_arrow
question_answer 78) At which temperature, the velocity of sound is double than that of \[0{}^\circ C\]:
A)
\[500{}^\circ C\]
done
clear
B)
\[819{}^\circ C\]
done
clear
C)
\[1071{}^\circ C\]
done
clear
D)
\[1430{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 79) In equilibrium, the velocity of molecules of a gas depends on its temperature as:
A)
\[\upsilon \propto T\]
done
clear
B)
\[\upsilon \propto \frac{1}{T}\]
done
clear
C)
\[\upsilon \propto \sqrt{T}\]
done
clear
D)
\[\upsilon \propto {{T}^{0}}\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following series, lie in visible spectrum?
A)
Lyman
done
clear
B)
Balmer
done
clear
C)
Paschen
done
clear
D)
found
done
clear
View Answer play_arrow
question_answer 81) Escape velocity of a body depends upon its mass as:
A)
\[{{\upsilon }_{e}}\propto m\]
done
clear
B)
\[{{\upsilon }_{e}}\propto \frac{1}{m}\]
done
clear
C)
\[{{\upsilon }_{e}}\propto \sqrt{m}\]
done
clear
D)
does not depend upon mass
done
clear
View Answer play_arrow
question_answer 82) Time period of a pendulum in a freely falling lift is:
A)
84.6 min
done
clear
B)
\[\infty \]
done
clear
C)
0
done
clear
D)
2 second
done
clear
View Answer play_arrow
question_answer 83) According to law of equal distribution of energy the mean energy of a molecule per degree of freedom is:
A)
\[\frac{1}{2}KT\]
done
clear
B)
\[KT\]
done
clear
C)
\[\frac{3}{2}KT\]
done
clear
D)
\[\frac{5}{2}KT\]
done
clear
View Answer play_arrow
question_answer 84) If momentum of a particle increased 30%, then increase in its kinetic energy is:
A)
30%
done
clear
B)
60%
done
clear
C)
69%
done
clear
D)
80%
done
clear
View Answer play_arrow
question_answer 85) If value of surface tension is 30 dyne cm then necessary work done in blowing a soap bubble from radius 2 cm to 4 cm will be:
A)
435 \[\pi \] erg
done
clear
B)
2880 \[\pi \]erg
done
clear
C)
1305\[\pi \] erg
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 86) Hertz is the unit of:
A)
frequency
done
clear
B)
intensity
done
clear
C)
momentum
done
clear
D)
wavelength
done
clear
View Answer play_arrow
question_answer 87) Orbital velocity of a planet revolving near the earth is:
A)
5 km/sec
done
clear
B)
8 km/sec
done
clear
C)
10.5 km/sec
done
clear
D)
11.2 km/sec
done
clear
View Answer play_arrow
question_answer 88) Dimension of Planck's constant is similar to:
A)
linear momentum
done
clear
B)
angular momentum
done
clear
C)
torque
done
clear
D)
velocity
done
clear
View Answer play_arrow
question_answer 89) Value of g at the surface of earth is \[10\,m/{{s}^{2}}\], then value of 'g' at a height \[R {{ }_{e}}\], from surface of earth is:
A)
\[1\,m/{{s}^{2}}\]
done
clear
B)
\[2.5\,m/{{s}^{2}}\]
done
clear
C)
\[4.5\,m/{{s}^{2}}\]
done
clear
D)
\[10\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 90) Two particles of same mass having kinetic energies 100 J and 400 J, then ratio of their momentum will be:
A)
2 : 1
done
clear
B)
1 : 2
done
clear
C)
4 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 91) Dimensions of h is:
A)
\[[{{M}^{1}}{{L}^{1}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{1}}{{L}^{2}}{{T}^{-1}}]\]
done
clear
C)
\[[{{M}^{1}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
D)
\[[{{M}^{1}}{{L}^{-2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 92) A particle moving with velocity r collides with a particle of same mass moving with the same velocity in opposite direction. If collision is perfectly elastic men velocity of the first particle after collision will be :
A)
zero
done
clear
B)
\[2\,\upsilon \]
done
clear
C)
\[\,\upsilon \]
done
clear
D)
\[3\,\upsilon \]
done
clear
View Answer play_arrow
question_answer 93) Transverse nature of light is examined by:
A)
dispersion
done
clear
B)
scattering
done
clear
C)
diffraction
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 94) Unit of Planck's constant in M.K.S. system is:
A)
Joule/sec
done
clear
B)
Joule-sec
done
clear
C)
erg/sec
done
clear
D)
volt
done
clear
View Answer play_arrow
question_answer 95) The angular velocity of a 20 cm rope if rotated in vertical ring, so that on highest point, its tension is zero:
A)
5 rad/sec
done
clear
B)
2 rad/sec
done
clear
C)
7.5 rad/sec
done
clear
D)
7 rad/sec
done
clear
View Answer play_arrow
question_answer 96) A body takes \[{{t}_{1}}\] and \[{{t}_{2}}\] time respectively if fall from heights \[{{h}_{1}}\] and \[{{h}_{2}}\], then ratio of \[{{t}_{1}}\] and \[{{t}_{2}}\] is:
A)
\[{{h}_{1}}:{{h}_{2}}\]
done
clear
B)
\[\sqrt{{{h}_{1}}}:\sqrt{{{h}_{2}}}\]
done
clear
C)
\[{{h}_{1}}:2{{h}_{2}}\]
done
clear
D)
\[2{{h}_{1}}:{{h}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) Two particles of mass 10 g and 40 g are moving with same kinetic energy, then ratio of linear momentum is:
A)
4 : 1
done
clear
B)
1 : 4
done
clear
C)
\[\sqrt{2}:1\]
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 98) Total internal reflection is possible when light ray:
A)
enters into water from air
done
clear
B)
enters into glass from air
done
clear
C)
enters into water from glass
done
clear
D)
enters into glass from water
done
clear
View Answer play_arrow
question_answer 99) If \[\hat{n}=a\hat{i}+b\hat{j},\] is perpendicular to (\[\hat{i}+\hat{j},\]then value of \[a\] and b is:
A)
1, 0
done
clear
B)
-2, 0
done
clear
C)
-3, 0
done
clear
D)
\[\frac{1}{\sqrt{2}},-\frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 100) \[\vec{a}\] and \[\vec{b}\] are such that \[|\vec{a}+\vec{b}|\,=\,|\vec{a}-\vec{b}\] then angle between \[\vec{a}\] and \[\vec{b}\] is:
A)
\[\pi /3\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\pi /2\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 101) Fermentation is:
A)
reversible
done
clear
B)
exothermic
done
clear
C)
endothermic
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 102) The ionization percentage of \[0.2M\] \[HCN\] the solution is \[0.02%\]. What will be the value of ionization constant:
A)
\[8\times {{10}^{-5}}\]
done
clear
B)
\[8\times {{10}^{-9}}\]
done
clear
C)
\[8\times {{10}^{-7}}\]
done
clear
D)
\[8\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 103) Which one act as Bronsted acid as well as Bronsted base?
A)
\[C{{l}^{-}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[HCl\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 104) If the pH of \[HCl\]solution is 2 then the molar concentration of this will be:
A)
\[0.01M\]
done
clear
B)
\[2M\]
done
clear
C)
\[0.4M\]
done
clear
D)
\[0.02M\]
done
clear
View Answer play_arrow
question_answer 105) \[B{{F}_{3}}\] molecule is:
A)
Lewis base
done
clear
B)
Lewis acid
done
clear
C)
Neutral
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 106) Which salt is insoluble in water?
A)
\[N{{H}_{4}}Cl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 107) Which compounds forms urotropine when reacts with \[N{{H}_{3}}\]?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 108) The compound formed by the ozonolysis of acetylene:
A)
glycol
done
clear
B)
acetic acid
done
clear
C)
ethylene ozonide
done
clear
D)
glyoxal
done
clear
View Answer play_arrow
question_answer 109) Number of electron in one molecule of \[N{{H}_{3}}\]:
A)
\[17\]
done
clear
B)
\[34\]
done
clear
C)
\[7\]
done
clear
D)
\[10\]
done
clear
View Answer play_arrow
question_answer 110) The values of magnetic quantum number for \[l=3\]:
A)
\[0,+1,+2,+3\]
done
clear
B)
\[0,\pm 1,\pm 2,\pm 3\]
done
clear
C)
\[-1,-2,-3\]
done
clear
D)
\[\pm 1,\pm 2,\pm 3\]
done
clear
View Answer play_arrow
question_answer 111) In the presence of ether, 2-chloropropane reacts with sodium and forms:
A)
n-hexane
done
clear
B)
2, 3 dimethyl butane
done
clear
C)
n-hexene
done
clear
D)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) The geometry of ammonia is:
A)
tetrahedral
done
clear
B)
pyramidal
done
clear
C)
triangular
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 113) Which one is colorless?
A)
\[C{{o}^{2+}}\]
done
clear
B)
\[N{{i}^{+2}}\]
done
clear
C)
\[F{{e}^{3+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 114) Which one have highest melting point
A)
\[CsCl\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[He\]
done
clear
D)
\[CHC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 115) Which one will not show Cannizzaro reaction?
A)
Trimethyl acetaldehyde
done
clear
B)
Formaldehyde
done
clear
C)
Acetaldehyde
done
clear
D)
Benzaldehyde
done
clear
View Answer play_arrow
question_answer 116) When \[23g\]sodium metal reacts with methyl alcohol, it will form:
A)
\[1mole\]of \[{{H}_{2}}\]
done
clear
B)
\[2\,mole\]of \[{{H}_{2}}\]
done
clear
C)
\[\frac{1}{2}mole\]of \[{{H}_{2}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 117) Which inorganic compound on heating changes into organic compound?
A)
Ammonium cyanide
done
clear
B)
Sodalime
done
clear
C)
Potassium cyanide
done
clear
D)
Sodamide
done
clear
View Answer play_arrow
question_answer 118) Which of the following compound will not show aldol condensation?
A)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 119) In first transition series, which element show maximum oxidation states:
A)
\[Co\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Cr\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 120) The bad odour compound formed by the reaction between chloroform and aniline in the presence of alcoholic \[KOH\]:
A)
acetylene
done
clear
B)
nitro benzene
done
clear
C)
phenyl isocyanate
done
clear
D)
chloromine
done
clear
View Answer play_arrow
question_answer 121) \[N{{O}_{2}}+\frac{1}{2}{{O}_{2}}N{{O}_{3}}...........{{K}_{1}}\] \[2N{{O}_{2}}+{{O}_{2}}2N{{O}_{3}}...........{{K}_{2}}\] For the above reaction the relation between equilibrium constant is:
A)
\[{{K}_{2}}={{({{K}_{1}})}^{2}}\]
done
clear
B)
\[{{K}_{2}}=\frac{1}{{{K}_{1}}}\]
done
clear
C)
\[{{K}_{2}}=\frac{1}{{{K}_{{{1}^{2}}}}}\]
done
clear
D)
\[{{K}_{1}}={{K}_{2}}\]
done
clear
View Answer play_arrow
question_answer 122) The condensation product of reaction between phenol and phthalic anhydride:
A)
methyl orange
done
clear
B)
salicylic acid
done
clear
C)
phenol red
done
clear
D)
phenolphthalein
done
clear
View Answer play_arrow
question_answer 123) Which characteristic property is not shown by carboxylic acid group?
A)
Alkyl group
done
clear
B)
\[-COOH\] group
done
clear
C)
group
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 124) The product of reaction between chloroform and acetone is used as:
A)
insecticide
done
clear
B)
analgesic
done
clear
C)
hypnotic
done
clear
D)
war gas
done
clear
View Answer play_arrow
question_answer 125) The strongest Bronsted base is:
A)
\[HCl{{O}_{2}}^{-}\]
done
clear
B)
\[HCl{{O}^{-}}\]
done
clear
C)
\[HCl{{O}_{3}}^{-}\]
done
clear
D)
\[HCl{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 126) The compound which have conjugate double bond:
A)
butyne
done
clear
B)
butylene
done
clear
C)
isobutylene
done
clear
D)
butadiene
done
clear
View Answer play_arrow
question_answer 127) Which salt on heating; does not give brown gas on heating?
A)
\[LiN{{O}_{3}}\]
done
clear
B)
\[AgN{{O}_{3}}\]
done
clear
C)
\[Pb{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[KN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 128) The product formed by the reaction between acetylene and \[HOCl\]:
A)
ethylene chloride
done
clear
B)
vinyl chloride
done
clear
C)
di chloro acetaldehyde
done
clear
D)
ethyledene chloride
done
clear
View Answer play_arrow
question_answer 129) The characteristic ion of an acid in an aqueous solution is:
A)
\[{{H}^{+}}\]
done
clear
B)
\[{{H}_{{{2}^{+}}}}\]
done
clear
C)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
D)
\[{{H}_{4}}{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 130) The most paramagnetic is:
A)
\[C{{r}^{3+}}\]
done
clear
B)
\[C{{O}^{2+}}\]
done
clear
C)
\[M{{n}^{2+}}\]
done
clear
D)
\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 131) The present undesirable matrix in mineral is called as:
A)
flux
done
clear
B)
slag
done
clear
C)
gangue
done
clear
D)
alloy
done
clear
View Answer play_arrow
question_answer 132) The compound with maximum covalent character is:
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[Al{{I}_{3}}\]
done
clear
C)
\[Ag{{I}_{2}}\]
done
clear
D)
\[NaI\]
done
clear
View Answer play_arrow
question_answer 133) \[N{{F}_{3}}\] and \[B{{F}_{3}}\]both are covalent, but \[B{{F}_{3}}\] is non-polar, while \[N{{F}_{3}}\] is polar because:
A)
Boron is non metal in free state, while \[{{N}_{2}}\]is gas
done
clear
B)
There is no dipole moment in \[B-F\]bond while \[N-F\]bond have some dipole moment.
done
clear
C)
\[B{{F}_{3}}\] is planar while \[N{{F}_{3}}\]is pyramidal
done
clear
D)
Boron atom is smaller than nitrogen atom
done
clear
View Answer play_arrow
question_answer 134) Many compounds are formed by carbon atom because of its:
A)
more reactivity
done
clear
B)
covalent and ionic nature
done
clear
C)
variable valency
done
clear
D)
catenation nature
done
clear
View Answer play_arrow
question_answer 135) For the complete combustion of 4 litre ethane, how much oxygen is required?
A)
\[14\text{ }litre\]
done
clear
B)
\[4\text{ }litre\]
done
clear
C)
\[\text{8 }litre\]
done
clear
D)
\[\text{12 }litre\]
done
clear
View Answer play_arrow
question_answer 136) In a group of periodic table what is the basis of:
A)
ionic potential
done
clear
B)
electron affinity
done
clear
C)
electron negativity
done
clear
D)
number of covalent electron
done
clear
View Answer play_arrow
question_answer 137) The work of cone. \[{{H}_{2}}S{{O}_{4}}\] in esterification process is as:
A)
dehydrating agent and catalyst
done
clear
B)
dehydrating agent
done
clear
C)
hydrolyzing agent
done
clear
D)
catalyst
done
clear
View Answer play_arrow
question_answer 138) When magnesium is heated, the another compound formed with \[MgO\] is:
A)
\[M{{g}_{3}}{{N}_{2}}\]
done
clear
B)
\[Mg{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[MgC{{O}_{3}}\]
done
clear
D)
\[Mg{{(N{{O}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 139) The \[C-H\] bond energy in ethane, ethene and ethyne:
A)
more in ethane
done
clear
B)
more in ethane
done
clear
C)
more in ethyne
done
clear
D)
same in all the above
done
clear
View Answer play_arrow
question_answer 140) The solubility of \[AgCl\] is \[1.435gm/litre\]. What will be the solubility product?
A)
\[{{10}^{-4}}\]
done
clear
B)
\[0.01\]
done
clear
C)
\[0.001\]
done
clear
D)
\[0.1\]
done
clear
View Answer play_arrow
question_answer 141) If two elements X and Y have 3 and 6 electrons in its outer most shell, then the molecular formula of compound formed by the combination of X and Y:
A)
\[XY\]
done
clear
B)
\[{{X}_{3}}Y~\]
done
clear
C)
\[{{X}_{2}}Y\]
done
clear
D)
\[{{X}_{2}}{{Y}_{3}}\]
done
clear
View Answer play_arrow
question_answer 142) The I.U.P.A.C. name of \[\underset{C{{H}_{2}}}{\overset{OH}{\mathop{|}}}\,.\underset{C{{H}_{2}}}{\overset{OH}{\mathop{|}}}\,\,\,\,\,.\,\,\,\underset{C{{H}_{2}}}{\overset{OH}{\mathop{|}}}\,\,\,\,\]is:
A)
1, 2, 3 propane tri-ol
done
clear
B)
3-hydroxy butanoic acid
done
clear
C)
1, 2 ethane di-ol
done
clear
D)
3-methyl butanol-1
done
clear
View Answer play_arrow
question_answer 143) The number of \[{{H}^{+}}\] ion in half normal solution of \[N{{a}_{2}}C{{O}_{3}}\]and \[C{{H}_{3}}COOH\]:
A)
\[>{{10}^{-7}}m\]
done
clear
B)
\[{{10}^{-7}}m\]
done
clear
C)
\[<{{10}^{-7}}m\]
done
clear
D)
\[{{10}^{-5}}m\]
done
clear
View Answer play_arrow
question_answer 144) Which of the following is not a redox reaction?
A)
\[KCN+Fe{{(CN)}_{2}}\xrightarrow{{}}{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
B)
\[Rb+{{H}_{2}}O\xrightarrow{{}}RbOH+{{H}_{2}}\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O+O\]
done
clear
D)
\[Cu{{I}_{2}}\xrightarrow{{}}CuI+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 145) The correct electronic configuration of element with atomic number 29:
A)
\[1{{s}^{2}},2{{s}^{2}}\text{ }2{{p}^{6}},3{{s}^{2}}\text{ }3{{p}^{6}}\text{ }3{{d}^{10}},4{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{11}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}}\text{ }3{{s}^{2}}\text{ }3{{p}^{17}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) Which of the following statement is correct for ketone and acetaldehyde?
A)
Both reacts with \[HCN\] and forms hydrine
done
clear
B)
Both reacts with \[NaOH\]and forms polymer
done
clear
C)
Both forms acid on oxidation and reduction
done
clear
D)
Both forms alcohol on reduction and oxidation
done
clear
View Answer play_arrow
question_answer 147) The prefix word 'alkali' for alkaline metals shows:
A)
lustre of silver
done
clear
B)
ashes of plant
done
clear
C)
metallic nature
done
clear
D)
active metal
done
clear
View Answer play_arrow
question_answer 148) \[2X+2Y\xrightarrow{{}}2Z\] The equilibrium constant for the above reaction:
A)
\[\frac{[2X]\,[2Y]}{[2Z]}\]
done
clear
B)
\[\frac{[X]\,[Y]}{[Z]}\]
done
clear
C)
\[\frac{\,{{[Z]}^{2}}}{{{[X]}^{2}}{{[Y]}^{2}}}\]
done
clear
D)
\[\frac{\,{{[Z]}^{2}}}{[X][Y]}\]
done
clear
View Answer play_arrow
question_answer 149) The solubility product for XY is:
A)
\[{{s}^{2}}\]
done
clear
B)
\[2s\]
done
clear
C)
\[4{{s}^{2}}\]
done
clear
D)
\[2s\]
done
clear
View Answer play_arrow
question_answer 150) Aldehyde and ketone can be differentiated by:
A)
sodium bi sulphite
done
clear
B)
Fehling solution
done
clear
C)
ammonia
done
clear
D)
sulphuric acid
done
clear
View Answer play_arrow
question_answer 151) The product of the reaction between chloral and \[NaOH\] is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 152) The correct reactivity order is:
A)
\[C{{H}_{3}}CHO>{{(C{{H}_{3}})}_{2}}CO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CO>C{{H}_{3}}CHO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[C{{H}_{3}}CHO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}>{{(C{{H}_{3}})}_{2}}CO\]
done
clear
D)
\[C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}>C{{H}_{3}}CHO>{{(C{{H}_{3}})}_{2}}CO\]
done
clear
View Answer play_arrow
question_answer 153) The example of condensation polymerization is:
A)
formaldehyde - met formaldehyde
done
clear
B)
acetaldehyde - para acetaldehyde
done
clear
C)
acetone - mesityl oxide
done
clear
D)
ethene - polyethene
done
clear
View Answer play_arrow
question_answer 154) The probability of finding of three impaired electron in nitrogen atom is defined by:
A)
Aufbau's principle
done
clear
B)
Uncertainty principle
done
clear
C)
Fault's principle
done
clear
D)
Hund's rule
done
clear
View Answer play_arrow
question_answer 155) In a reversible reaction if the concentration of reactants and products are doubled, the value of \[{{K}_{c}}\]will be:
A)
half of the initial value
done
clear
B)
double of initial value
done
clear
C)
one fourth of the initial value
done
clear
D)
same the initial value
done
clear
View Answer play_arrow
question_answer 156) The main product of heating of sodium formate at \[{{360}^{o}}C\]is:
A)
\[CO\]
done
clear
B)
sodium oxalate
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) The number of \[\sigma \] (sigma) and \[\pi \] (pi) bonds in 1-butene-3-yne is:
A)
\[5\sigma ,5\pi \]
done
clear
B)
\[8\sigma ,6\pi \]
done
clear
C)
\[7\sigma ,3\pi \]
done
clear
D)
\[6\sigma ,4\pi \]
done
clear
View Answer play_arrow
question_answer 158) The correct size of iodine atom and iodine ion is:
A)
\[{{I}^{-}}>{{I}^{+}}+I\]
done
clear
B)
\[I>{{I}^{+}}+{{I}^{-}}\]
done
clear
C)
\[I>{{I}^{-}}+{{I}^{+}}\]
done
clear
D)
\[{{I}^{+}}>I+{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 159) The geometry of the compound of \[s{{p}^{3}}d\] hybridisation:
A)
pyramidal
done
clear
B)
triangular
done
clear
C)
planar
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 160) Potassium nitrate is called:
A)
Mohr's salt
done
clear
B)
Chily salt petre
done
clear
C)
Indian salt petre
done
clear
D)
Gypsum
done
clear
View Answer play_arrow
question_answer 161) The value of \[{{K}_{h}}\] for salt of weak acid and weak base is:
A)
\[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}}\]
done
clear
B)
\[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}.{{K}_{b}}}\]
done
clear
C)
\[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{h}}}\]
done
clear
D)
hydrolysis does not occur
done
clear
View Answer play_arrow
question_answer 162) Which one is required for the separation of proton from nucleus?
A)
Nucleus energy
done
clear
B)
Thermal energy
done
clear
C)
Binding energy
done
clear
D)
Kinetic energy
done
clear
View Answer play_arrow
question_answer 163) The compound formed by the reaction of ethyl-bromide and alcoholic \[KOH\] is:
A)
ethylene
done
clear
B)
acetylene
done
clear
C)
ethane
done
clear
D)
ethyl alcohol
done
clear
View Answer play_arrow
question_answer 164) The aqueous solution \[C{{H}_{3}}COONa\]will be:
A)
weak alkaline
done
clear
B)
acidic
done
clear
C)
strong alkaline
done
clear
D)
neutral
done
clear
View Answer play_arrow
question_answer 165) The formation of cyanohydrin from \[C{{H}_{3}}COC{{H}_{3}}\]is called:
A)
electrophilic substitution
done
clear
B)
nucleophilic addition
done
clear
C)
nucleophilic substitution
done
clear
D)
electrophilic addition
done
clear
View Answer play_arrow
question_answer 166) Passed in red hot iron of acetylene gives:
A)
benzene
done
clear
B)
vinyl acetylene
done
clear
C)
ethane
done
clear
D)
ethelene
done
clear
View Answer play_arrow
question_answer 167) The pair of compounds with maximum and minimum ionic character for \[LiCl\], \[RbCl,\] \[BeC{{l}_{2}}\]and \[MgC{{l}_{2}}\]:
A)
\[LiCI,MgC{{l}_{2}}\]
done
clear
B)
\[RbCl,MgC{{l}_{2}}\]
done
clear
C)
\[RbCl,BeC{{l}_{2}}\]
done
clear
D)
\[MgC{{l}_{2}},BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 168) Which of the following compound obtained by Williamson's synthesis?
A)
Ether
done
clear
B)
Alcohol
done
clear
C)
Alkylhalide
done
clear
D)
Ketone
done
clear
View Answer play_arrow
question_answer 169) The scientist responsible for the charge of electron is:
A)
Coulomb
done
clear
B)
Thomson
done
clear
C)
Chadwik
done
clear
D)
Milikan
done
clear
View Answer play_arrow
question_answer 170) At equilibrium, \[HCl\] is added in \[NaCl\] solution, \[NaCl\] precipitates, because:
A)
\[HCl\] is strong acid
done
clear
B)
\[HCl\] is weak acid
done
clear
C)
the solubility of \[NaCl\] decreases
done
clear
D)
ionic product of \[NaCl\] is more than \[{{K}_{sp}}\]
done
clear
View Answer play_arrow
question_answer 171) In which compound addition of \[HBr\] does not show according to Anti Markownikoff's rule?
A)
Benzene
done
clear
B)
2-pentene
done
clear
C)
2-butene
done
clear
D)
Propene
done
clear
View Answer play_arrow
question_answer 172) Lithopone is:
A)
\[Bas+ZnS{{O}_{4}}\]
done
clear
B)
\[Zns+CaS{{O}_{4}}\]
done
clear
C)
\[BaS{{O}_{4}}+ZnS\]
done
clear
D)
\[MgS{{O}_{4}}+ZnS\]
done
clear
View Answer play_arrow
question_answer 173) Which one is not ore of iron?
A)
Limonite
done
clear
B)
Caeseterite
done
clear
C)
Sedarite
done
clear
D)
Magnetite
done
clear
View Answer play_arrow
question_answer 174) Which isomerism is not shown by ether?
A)
Functional isomerism
done
clear
B)
Position isomerism
done
clear
C)
Metamerism
done
clear
D)
Chain isomerism
done
clear
View Answer play_arrow
question_answer 175) Least basic is:
A)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}}-C{{H}_{2}})}_{2}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 176) The metal present in chlorophyll is:
A)
\[Fe\]
done
clear
B)
\[Ni\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Zn\]
done
clear
View Answer play_arrow
question_answer 177) In which of following compound has not \[+6\] oxidation number of S?
A)
\[S{{O}_{3}}\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[S{{O}_{4}}^{-\,\,-}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 178) The correct order of acidity of acetic acid sulphuric acid and carbonic acid is:
A)
\[C{{H}_{3}}COOH<{{H}_{2}}S{{O}_{4}}<{{H}_{2}}C{{O}_{3}}\]
done
clear
B)
\[{{H}_{2}}C{{O}_{3}}<C{{H}_{3}}COOH<{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[C{{H}_{3}}COOH<{{H}_{2}}C{{O}_{3}}<{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}<{{H}_{2}}C{{O}_{3}}<C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 179) In which compound the rotation of carbon-carbon bond is minimum?
A)
Ethylene
done
clear
B)
Acetylene
done
clear
C)
Hexa chloro ethane
done
clear
D)
Ethane
done
clear
View Answer play_arrow
question_answer 180) Acetamide is:
A)
acidic
done
clear
B)
amphoteric
done
clear
C)
basic
done
clear
D)
neutral
done
clear
View Answer play_arrow
question_answer 181) In which compound \[Mn\] has minimum oxidation state?
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[Mn{{O}_{2}}\]
done
clear
C)
\[{{K}_{2}}Mn{{O}_{4}}\]
done
clear
D)
\[M{{n}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 182) Which one have maximum magnetic momentum?
A)
\[3{{d}^{5}}\]
done
clear
B)
\[3{{d}^{2}}\]
done
clear
C)
\[3{{d}^{9}}\]
done
clear
D)
\[3{{d}^{7}}\]
done
clear
View Answer play_arrow
question_answer 183) \[2A+2B2C+2D\] For the above equation, the correct relationship is:
A)
\[{{K}_{p}}={{K}_{c}}{{(RT)}^{-2}}\]
done
clear
B)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}=\frac{{{K}_{c}}}{2}\]
done
clear
D)
\[{{K}_{p}}={{K}_{c}}{{(RT)}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 184) The nitration of benzene is:
A)
electrophilic addition
done
clear
B)
electrophilic substitution
done
clear
C)
nucleophilic addition
done
clear
D)
nucleophilic substitution
done
clear
View Answer play_arrow
question_answer 185) Benzene shows:
A)
substitution
done
clear
B)
oxidation
done
clear
C)
addition
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 186) An electronic configuration of a metal is 2, 8, 14 and mass number of metal is 56. Then the number of neutron in its nucleus is:
A)
\[32\]
done
clear
B)
\[30\]
done
clear
C)
\[34\]
done
clear
D)
\[42\]
done
clear
View Answer play_arrow
question_answer 187) At room temperature formaldehyde is:
A)
liquid
done
clear
B)
solid
done
clear
C)
gas
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 188) The highest \[C-H\]bond energy in:
A)
ethene
done
clear
B)
ethyne
done
clear
C)
ethane
done
clear
D)
equal in all
done
clear
View Answer play_arrow
question_answer 189) Formic acid and acetic acid can be distinguished chemically by:
A)
reaction with \[HCl\]
done
clear
B)
iodoform test
done
clear
C)
reaction with \[N{{H}_{3}}\]
done
clear
D)
reaction with \[FeC{{l}_{3}}\] solution
done
clear
View Answer play_arrow
question_answer 190) \[CHC\equiv CH\xrightarrow[Ni]{{{H}_{2}}}A,\] product A is:
A)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 191) \[PC{{l}_{5}}PC{{l}_{3}}+C{{l}_{2}}\]in this reversible reaction the moles of \[PC{{l}_{5}},\] \[PC{{l}_{3}},\] and \[C{{l}_{2}}\] are a, b and c respectively and total pressure is P then value of \[{{K}_{p}}\] is:
A)
\[\frac{bc}{a}.RT\]
done
clear
B)
\[\frac{b}{(a+b+c)}.P\]
done
clear
C)
\[\frac{bc.P}{a(a+b+c)}\]
done
clear
D)
\[\frac{c}{(a+b+c)}.P\]
done
clear
View Answer play_arrow
question_answer 192) \[\underset{\begin{smallmatrix} || \\ C{{H}_{2}} \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\xrightarrow{{{O}_{3}}}X\xrightarrow{{{H}_{2}}O}Y+Z.\] In this reaction Y compound is:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}OH\]
done
clear
D)
\[CH{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 193) The correct reactivity order of alkyl halide is:
A)
\[RI>RCl>RBr\]
done
clear
B)
\[RCl>RI>RBr\]
done
clear
C)
\[RCl>RBr>RI\]
done
clear
D)
\[RI>RBr>RCl\]
done
clear
View Answer play_arrow
question_answer 194) The compound obtained by the reaction between urea and hydrazine is:
A)
barbituric acid
done
clear
B)
methyl urea
done
clear
C)
semi carbide
done
clear
D)
acetamide
done
clear
View Answer play_arrow
question_answer 195) Which indicator used in titration of sodium carbonate and \[HCl\]?
A)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
B)
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
Phenolphthalien
done
clear
D)
Methyl orange
done
clear
View Answer play_arrow
question_answer 196) Which of the following set of four quantum number is not allowed for an electron of an atom?
A)
\[n=1,\,l=0,\,m=1,s=+\frac{1}{2}\]
done
clear
B)
\[n=1,\,l=1,\,m=1,s=+\frac{1}{2}\]
done
clear
C)
\[n=1,\,l=0,\,m=1,s=-\frac{1}{2}\]
done
clear
D)
\[n=2,\,l=1,\,m=1,s=+\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 197) If two ice cube are in contact, pressure is exerted, which is responsible to joint the two ice cubes?
A)
Hydrogen bond
done
clear
B)
Covalent bond
done
clear
C)
van der Waal's bond
done
clear
D)
Dipole attraction force
done
clear
View Answer play_arrow
question_answer 198) \[Mn{{O}_{4}}+{{C}_{2}}O_{4}^{2-}+{{H}^{+}}\xrightarrow{{}}M{{n}^{2+}}\] \[+C{{O}_{2}}+{{H}_{2}}O\] In the above reaction the number of reactants respectively:
A)
\[2, 5, 16\]
done
clear
B)
\[16, 3, 2\]
done
clear
C)
\[2, 16, 5\]
done
clear
D)
\[5, 16, 2\]
done
clear
View Answer play_arrow
question_answer 199) Operator genes are controls to which gene mechanism?
A)
Structural gene
done
clear
B)
Activator gene
done
clear
C)
Regulator gene
done
clear
D)
Modulac gene
done
clear
View Answer play_arrow
question_answer 200) How many ovum are developed in ovary of Hydra?
A)
Two
done
clear
B)
Many
done
clear
C)
One
done
clear
D)
Three
done
clear
View Answer play_arrow
question_answer 201) Respiration in Ascaris is:
A)
aerobic respiration
done
clear
B)
anaerobic respiration
done
clear
C)
both 'a' and 'b'
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 202) Type of blastula in chik is :
A)
discoblastula
done
clear
B)
coeloblastula
done
clear
C)
amphiblastula
done
clear
D)
holoblastula
done
clear
View Answer play_arrow
question_answer 203) Respiration pigment of blood in Cockroach is:
A)
haemozoine
done
clear
B)
haemocyanin
done
clear
C)
haemoglobin
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 204) Which organ is enlarged in malarial patient?
A)
Spleen
done
clear
B)
Kidney
done
clear
C)
Gall bladder
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 205) Golgi body is:
A)
organ for protein synthesis
done
clear
B)
secretory organ
done
clear
C)
both 'a' and 'b'
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 206) DNA is specific because it has :
A)
numbers of nucleotides
done
clear
B)
specific nature of phosphate and sugar
done
clear
C)
arrangement of protein in DNA
done
clear
D)
specific nature of purine and pyrimidines
done
clear
View Answer play_arrow
question_answer 207) Role of carbohydrate in protoplasm :
A)
as catalyst
done
clear
B)
for energy
done
clear
C)
as enzymes
done
clear
D)
for synthesis
done
clear
View Answer play_arrow
question_answer 208) In Amoeba hyaline cap is formed by :
A)
around the food vacuole
done
clear
B)
around the contractile vacuole
done
clear
C)
around the nucleus
done
clear
D)
on pseudopodia
done
clear
View Answer play_arrow
question_answer 209) Which tissue covered with calcium phosphate?
A)
Bone
done
clear
B)
Muscles
done
clear
C)
Mesenchyma
done
clear
D)
Cartilage
done
clear
View Answer play_arrow
question_answer 210) Lamp brush chromosomes are present in:
A)
Drosophilla
done
clear
B)
Ascaris
done
clear
C)
Hydra
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 211) Comb jelly is member of which of phylum?
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Coelenterata
done
clear
D)
Ctenophora
done
clear
View Answer play_arrow
question_answer 212) Jaws present in which of the following?
A)
Heradmania
done
clear
B)
Fishes
done
clear
C)
Pctromyzon
done
clear
D)
Amphwxus
done
clear
View Answer play_arrow
question_answer 213) Which of the following is not insect?
A)
Spider
done
clear
B)
Grasshopper
done
clear
C)
Fly
done
clear
D)
Lepisma
done
clear
View Answer play_arrow
question_answer 214) Microlecithal eggs are present in which animals?
A)
Insects
done
clear
B)
Aves
done
clear
C)
Fishes
done
clear
D)
Mammals
done
clear
View Answer play_arrow
question_answer 215) Muscular movements of alimentary canal are known as :
A)
peristalsis
done
clear
B)
diastole
done
clear
C)
systole
done
clear
D)
muscles contraction
done
clear
View Answer play_arrow
question_answer 216) From acrosome which secrets:
A)
hyaluronic acid
done
clear
B)
hyaluronidase
done
clear
C)
TSH
done
clear
D)
fertilizing
done
clear
View Answer play_arrow
question_answer 217) When embryonic development is completed in mother but the embryo not accept nutritent from mother. Which is known as:
A)
ovoviparous
done
clear
B)
viviparous
done
clear
C)
oviparous
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 218) Gastrula is differ from blastula in:
A)
three germ layers
done
clear
B)
micromeres
done
clear
C)
blastocoel
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 219) Which gland is worked opposite to pressure?
A)
Adrenal
done
clear
B)
Parathyroid
done
clear
C)
Pineal
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
question_answer 220) First heart sound is produced during closure of:
A)
auriculo-ventricular valves
done
clear
B)
eustechian valve
done
clear
C)
sinus valve
done
clear
D)
seminular valves
done
clear
View Answer play_arrow
question_answer 221) In motor neuron the impulse following in which direction?
A)
In two direction
done
clear
B)
In one direction
done
clear
C)
In all direction
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 222) Nucleated RBCs are present in which?
A)
Rabbit
done
clear
B)
Camel and Lama
done
clear
C)
Embryo of human
done
clear
D)
Both 'b' and 'c'
done
clear
View Answer play_arrow
question_answer 223) Sertoli cells are associated with :
A)
kidney of Rabbit
done
clear
B)
ovary of Frog
done
clear
C)
testis of Rabbit
done
clear
D)
ovary of Rabbit
done
clear
View Answer play_arrow
question_answer 224) Final excretion in urine of which substance is :
A)
amino acid
done
clear
B)
urea
done
clear
C)
glucose and glycogen
done
clear
D)
uric acid
done
clear
View Answer play_arrow
question_answer 225) Haemoglobin in Earthworm is present in:
A)
RBC
done
clear
B)
protoplasm
done
clear
C)
coelomic fluid
done
clear
D)
plasma
done
clear
View Answer play_arrow
question_answer 226) Gametes formation in animals is found in;
A)
ovary
done
clear
B)
gonads
done
clear
C)
gall bladder
done
clear
D)
testis
done
clear
View Answer play_arrow
question_answer 227) Fertilization in Earthworm is :
A)
cross fertilization
done
clear
B)
combined fertilization
done
clear
C)
self-fertilization
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 228) Hydra is:
A)
herbivorous
done
clear
B)
more developed
done
clear
C)
carnivorous
done
clear
D)
omnivorous
done
clear
View Answer play_arrow
question_answer 229) Which pigment is cause of malaria?
A)
haemocyanine
done
clear
B)
haemoglobin
done
clear
C)
haemozoine
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 230) Changing of a small Cockroach into adults is know as:
A)
moulting
done
clear
B)
metamorphosis
done
clear
C)
ecdysis
done
clear
D)
transformation
done
clear
View Answer play_arrow
question_answer 231) Cockroach is an animal of which class?
A)
Hexapoda
done
clear
B)
Apoda
done
clear
C)
Meriopda
done
clear
D)
Cephalopoda
done
clear
View Answer play_arrow
question_answer 232) Formation of pseudopodia due to:
A)
chemical changes
done
clear
B)
viscosity changes
done
clear
C)
difference in pressure
done
clear
D)
changing in temperature
done
clear
View Answer play_arrow
question_answer 233) Hydra is:
A)
triploblastic, radial symmetrical and acoelomate
done
clear
B)
triploblastic, radial symmetrical and coelomate
done
clear
C)
diplobalstic, radial symmetrical and acoelomate
done
clear
D)
diploblastic, radial symmetrical and coelomate
done
clear
View Answer play_arrow
question_answer 234) Malignant malaria is cause by :
A)
Plasmodium falciparum
done
clear
B)
Plasmodium ovale
done
clear
C)
Plasmodium vivax
done
clear
D)
Plasmodium malariae
done
clear
View Answer play_arrow
question_answer 235) Adenine is :
A)
pyrimidine base
done
clear
B)
purine base
done
clear
C)
five carbon ring
done
clear
D)
structure with ring
done
clear
View Answer play_arrow
question_answer 236) \[1\,\mu \] is equal to:
A)
1 /1000 mm
done
clear
B)
1 /100 mm
done
clear
C)
\[{{10}^{-9}}mm\]
done
clear
D)
\[{{10}^{-6}}mm\]
done
clear
View Answer play_arrow
question_answer 237) Defect in gene duplication is known as :
A)
transformation
done
clear
B)
transduction
done
clear
C)
mutation
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 238) Cell division is :
A)
S - phase
done
clear
B)
\[{{G}_{2}}\]- phase
done
clear
C)
\[{{G}_{1}}\]- phase
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 239) RBC volume increase when put in :
A)
isotonic
done
clear
B)
protoplasm
done
clear
C)
hypertonic
done
clear
D)
hypotonic
done
clear
View Answer play_arrow
question_answer 240) Which Frog is found on trees?
A)
Altes
done
clear
B)
Bufo
done
clear
C)
Hyla
done
clear
D)
Rana
done
clear
View Answer play_arrow
question_answer 241) Class of insecta is:
A)
Decapoda
done
clear
B)
Octopoda
done
clear
C)
Hexapoda
done
clear
D)
Apoda
done
clear
View Answer play_arrow
question_answer 242) Paired vagina present in:
A)
Monotrimata
done
clear
B)
Eutheria
done
clear
C)
Marsupiales
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 243) Outer covering of tunicates is formed by:
A)
tunicin
done
clear
B)
cellulose
done
clear
C)
chitin
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 244) Connecting link between bird and Reptiles is:
A)
Dodo
done
clear
B)
Kiwi
done
clear
C)
Panguine
done
clear
D)
Archaeopteryx
done
clear
View Answer play_arrow
question_answer 245) Fishes are:
A)
homiothermal
done
clear
B)
poikilothermal
done
clear
C)
both 'a' and 'b'
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 246) The most relativity of CO than \[C{{O}_{2}}\] in which compound ?
A)
Carboxy haemoglobin
done
clear
B)
Oxygen
done
clear
C)
oxy haemoglobin
done
clear
D)
Haemoglobin
done
clear
View Answer play_arrow
question_answer 247) Dimorphism is present in which Protozoa ?
A)
lobosa
done
clear
B)
Ciliata
done
clear
C)
Sporozoans
done
clear
D)
Radiolarian
done
clear
View Answer play_arrow
question_answer 248) Structural and function unit of kidney is :
A)
nephrone
done
clear
B)
efferent arteriole
done
clear
C)
glornerulus
done
clear
D)
neuron
done
clear
View Answer play_arrow
question_answer 249) Medulla - oblongata is controls of:
A)
blood pressure
done
clear
B)
smell or odour
done
clear
C)
high temperature
done
clear
D)
low temperature
done
clear
View Answer play_arrow
question_answer 250) Infective stage of Ascaris is :
A)
embryonated eggs
done
clear
B)
sporozoite
done
clear
C)
cysticercus larva
done
clear
D)
rhabditiform larva
done
clear
View Answer play_arrow
question_answer 251) In Hydra, which substance of nematocyst worked as hipnotizing substance?
A)
Glutatheone
done
clear
B)
Heparin
done
clear
C)
Histamin
done
clear
D)
Hypnotoxine
done
clear
View Answer play_arrow
question_answer 252) Which stage is comes just after cleavage?
A)
Invagination
done
clear
B)
Evagination
done
clear
C)
Gastrula
done
clear
D)
Blastula
done
clear
View Answer play_arrow
question_answer 253) Capsulogenous gland is present in :
A)
ovary
done
clear
B)
cocoon
done
clear
C)
spermatheca
done
clear
D)
collnterial gland
done
clear
View Answer play_arrow
question_answer 254) All mechanism of living organism divided in two parts:
A)
reproduction and evolution
done
clear
B)
nutrition and metabolism
done
clear
C)
synthesis and metabolism
done
clear
D)
reproduction and internal autonomy
done
clear
View Answer play_arrow
question_answer 255) When the particles are distributed freely in colloid then it called :
A)
sol
done
clear
B)
suspension matter
done
clear
C)
emulsion
done
clear
D)
gel
done
clear
View Answer play_arrow
question_answer 256) Sucking of liquids from surface by cell known as:
A)
diffusion
done
clear
B)
phagocytosis
done
clear
C)
pinocytosis
done
clear
D)
osmosis
done
clear
View Answer play_arrow
question_answer 257) Life start from which substance to upper?
A)
molecule
done
clear
B)
amino acid
done
clear
C)
mixture substance
done
clear
D)
complex
done
clear
View Answer play_arrow
question_answer 258) Which type of image is found in eye of Cockroach ?
A)
Mosaic
done
clear
B)
Superposition
done
clear
C)
Overlapping
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 259) Broad description of Amoeba given by:
A)
Ronald Ross
done
clear
B)
Aristotle
done
clear
C)
Harshfeild
done
clear
D)
Rosenholf
done
clear
View Answer play_arrow
question_answer 260) In Amoeba binary fission is by :
A)
amitosis
done
clear
B)
mitosis
done
clear
C)
meiosis
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 261) Which of the following animals are not present in fresh water?
A)
Crustacea
done
clear
B)
Insecta
done
clear
C)
Echinodermata
done
clear
D)
Sponges
done
clear
View Answer play_arrow
question_answer 262) Elephant tusk shell is:
A)
chitin
done
clear
B)
nautilus
done
clear
C)
patella
done
clear
D)
dentalium
done
clear
View Answer play_arrow
question_answer 263) Aristotle lantern is present in:
A)
Antedon
done
clear
B)
Solaster
done
clear
C)
Sea-urchin
done
clear
D)
Sea-cucumber
done
clear
View Answer play_arrow
question_answer 264) Retrogressive metamorphosis is present in
A)
Herdmania
done
clear
B)
Balanoglossus
done
clear
C)
Amphioxus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 265) Which of the following class of Protozoa is totally class of parasites?
A)
Mestigophora
done
clear
B)
Ciliata
done
clear
C)
Sporozoa
done
clear
D)
Rhizopoda
done
clear
View Answer play_arrow
question_answer 266) Dicondylic stage and ten pairs of cranial 'nerves are present in following?
A)
Reptilia
done
clear
B)
Aves
done
clear
C)
Amphibia
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 267) Which vitamin is formed by skin?
A)
Vitamin - B
done
clear
B)
Vitamin - E
done
clear
C)
Vitamin - D
done
clear
D)
Vitamin - A
done
clear
View Answer play_arrow
question_answer 268) Corpus leuteum secrets:
A)
LH
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 269) Urea is synthesized in:
A)
liver
done
clear
B)
intestine
done
clear
C)
stomach
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 270) Respiratory centre is present where:
A)
cerebrum
done
clear
B)
hypothalamus
done
clear
C)
cerebellum
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 271) Which of the following Sponge is present in river?
A)
Cliona
done
clear
B)
Spongilla
done
clear
C)
Sycon
done
clear
D)
Hyalonema
done
clear
View Answer play_arrow
question_answer 272) Which structure is formed by keratin?
A)
Dermal papilla
done
clear
B)
Hair shaft
done
clear
C)
Root of hair
done
clear
D)
Hair follicle
done
clear
View Answer play_arrow
question_answer 273) Origin of nervous system from :
A)
meso-endoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
ectoderm
done
clear
View Answer play_arrow
question_answer 274) Thyroxin is secreted by which gland?
A)
Adrenal
done
clear
B)
Parathyroid
done
clear
C)
Pituitary
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
question_answer 275) Which substance is secreted by corpus leuteum?
A)
Hormone
done
clear
B)
Unzyme
done
clear
C)
Pheromone
done
clear
D)
Bile
done
clear
View Answer play_arrow
question_answer 276) Segments of Earthworm are called :
A)
metamere
done
clear
B)
sarcomere
done
clear
C)
prostomium
done
clear
D)
podomeres
done
clear
View Answer play_arrow
question_answer 277) Zymogenic cell is present in:
A)
corpora adipose
done
clear
B)
salivary gland of Cockroach
done
clear
C)
gizzard of Cockroach
done
clear
D)
compound eye of Cockroach
done
clear
View Answer play_arrow
question_answer 278) Penial setae are present in male Ascaris:
A)
in cloaca
done
clear
B)
in rectum
done
clear
C)
anus
done
clear
D)
mouth
done
clear
View Answer play_arrow
question_answer 279) Larval stage of Hydra is :
A)
planula
done
clear
B)
rhabditiform
done
clear
C)
trochophore
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 280) Female reproductive organ of Ascaris are :
A)
protandrous
done
clear
B)
polydelphic
done
clear
C)
didelphic
done
clear
D)
monodelphic
done
clear
View Answer play_arrow
question_answer 281) Which parasite is present in seminal vessicle of Earth worm?
A)
Monocystis
done
clear
B)
Nosema
done
clear
C)
Sarcocystis
done
clear
D)
Nictotherus
done
clear
View Answer play_arrow
question_answer 282) Malarial parasite harmful for liver cells in which cycle?
A)
Erythrocytic cycle
done
clear
B)
After erythrocytic cycle
done
clear
C)
Before erythrocytic cycle
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 283) Ascaris is completed its life cycle :
A)
only in Human
done
clear
B)
Human and Sheep
done
clear
C)
Human and Mosquito
done
clear
D)
Human and Cow
done
clear
View Answer play_arrow
question_answer 284) Malarial parasite is observed by :
A)
Sir Ronald Ross
done
clear
B)
Laveran
done
clear
C)
Patric Mantion
done
clear
D)
Grassi
done
clear
View Answer play_arrow
question_answer 285) Ribunucleoprotein particles of protoplasm are:
A)
ribosomes
done
clear
B)
plastid
done
clear
C)
golgi body
done
clear
D)
cristae
done
clear
View Answer play_arrow
question_answer 286) Which cell is without nucleus?
A)
Erythrocyte
done
clear
B)
Leucocytes
done
clear
C)
Muscles cells
done
clear
D)
Bone cells
done
clear
View Answer play_arrow
question_answer 287) Endoplasmic reticulum is absent in which?
A)
Monocytes of human
done
clear
B)
WBC of human
done
clear
C)
Embryonic cells
done
clear
D)
RBC of mammals
done
clear
View Answer play_arrow
question_answer 288) Function of contractile vacuole in Amoeba is:
A)
osmoregulation
done
clear
B)
nutrition
done
clear
C)
respiration
done
clear
D)
excretion
done
clear
View Answer play_arrow
question_answer 289) Main function of ribosomes is :
A)
fat synthesis
done
clear
B)
sterol synthesis
done
clear
C)
protein synthesis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 290) Which bird of the following is viviparous ?
A)
Panguine
done
clear
B)
Humming bird
done
clear
C)
Albatross
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 291) Snake felt sound from :
A)
tympanum
done
clear
B)
body
done
clear
C)
internal ear
done
clear
D)
earth
done
clear
View Answer play_arrow
question_answer 292) Fiberous tissue which connect bone to bone is:
A)
ligaments
done
clear
B)
cartilage
done
clear
C)
connective tissue
done
clear
D)
tendon
done
clear
View Answer play_arrow
question_answer 293) Deltoid groove is present in :
A)
redio-ulna
done
clear
B)
femur
done
clear
C)
tibio-fibula
done
clear
D)
humerus
done
clear
View Answer play_arrow
question_answer 294) Pneumatophore is helpful in:
A)
feeding
done
clear
B)
reproduction
done
clear
C)
defence
done
clear
D)
floating
done
clear
View Answer play_arrow
question_answer 295) Which nerves arise from spinal cord are?
A)
Mixed nerves
done
clear
B)
Motor
done
clear
C)
Sensory
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 296) Iris of eve sensitive for :
A)
light
done
clear
B)
chemical
done
clear
C)
heal
done
clear
D)
all
done
clear
View Answer play_arrow
question_answer 297) Main character of phylum Porifera is:
A)
canal system
done
clear
B)
water vascular system
done
clear
C)
reproductive system
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 298) Which of the following is shift in chloride-shift?
A)
\[{{O}_{2}}\] and\[C{{O}_{2}}\]
done
clear
B)
Bicarbonate ions
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 299) The pH of protoplasm is:
A)
6.0
done
clear
B)
6.8
done
clear
C)
7.8
done
clear
D)
8.2
done
clear
View Answer play_arrow
question_answer 300) The maximum element found in cytoplasm is:
A)
C
done
clear
B)
He
done
clear
C)
H
done
clear
D)
N
done
clear
View Answer play_arrow
question_answer 301) The enzymes found in lysosome are:
A)
hydrolytic enzymes
done
clear
B)
proteases enzymes
done
clear
C)
lipases enzymes
done
clear
D)
cellulases enzymes
done
clear
View Answer play_arrow
question_answer 302) Chromosome was discovered by:
A)
Hofmeister
done
clear
B)
Muller
done
clear
C)
Flemming
done
clear
D)
Hammerling
done
clear
View Answer play_arrow
question_answer 303) Insectivorous plant is:
A)
Cuscuta
done
clear
B)
Drosera
done
clear
C)
Striga
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 304) Mycoplasma is resistant for:
A)
penicillin
done
clear
B)
tetracycline
done
clear
C)
streptomycin
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 305) The reduction division in chromosomes takes place in:
A)
pollen grains
done
clear
B)
tapetum
done
clear
C)
megaspore cell
done
clear
D)
megaspore mother cell
done
clear
View Answer play_arrow
question_answer 306) Calvin cycle takes place in:
A)
grana
done
clear
B)
stroma
done
clear
C)
intra thylakoid
done
clear
D)
matrix
done
clear
View Answer play_arrow
question_answer 307) The hormone which prevents abscission is:
A)
IBA
done
clear
B)
ABA
done
clear
C)
K-N
done
clear
D)
GA
done
clear
View Answer play_arrow
question_answer 308) 'Sagopalm' is obtained from:
A)
Pinus
done
clear
B)
Cycas
done
clear
C)
Ginkgo
done
clear
D)
Date palm
done
clear
View Answer play_arrow
question_answer 309) Which of the following is not the function of 'golgi body??
A)
Protein synthesis
done
clear
B)
Formation of 'cell wall
done
clear
C)
Formation of fatty acids
done
clear
D)
Formation of plasma membrane
done
clear
View Answer play_arrow
question_answer 310) The RNA found in eukaryotic ribosome are:
A)
5S and 16S RNA
done
clear
B)
5S, 16S, 185 RNA
done
clear
C)
23S, 16S, RNA
done
clear
D)
5S and 28S RNA
done
clear
View Answer play_arrow
question_answer 311) Water is not necessary for the fertilization of:
A)
algae
done
clear
B)
bryophyta
done
clear
C)
Cycas
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 312) The biomass pyramid of forest ecosystem is:
A)
linear
done
clear
B)
inverted
done
clear
C)
tetra angle
done
clear
D)
upright
done
clear
View Answer play_arrow
question_answer 313) After pollination the number of chromosome in egg. will be:
A)
n
done
clear
B)
2n
done
clear
C)
3n
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 314) Double fertilization is the:
A)
fertilization of two eggs in an embryo sac
done
clear
B)
fusion of one male gamete with the egg cell and another male gamete with the secondary nuclei
done
clear
C)
fusion of two male gametes with two polar nuclei
done
clear
D)
fusion of one male gamete with secondary nucleus
done
clear
View Answer play_arrow
question_answer 315) The significance of mitochondria was given by:
A)
Flemming
done
clear
B)
Meves
done
clear
C)
Mendel
done
clear
D)
Altman
done
clear
View Answer play_arrow
question_answer 316) Mitochondria contains:
A)
only DNA
done
clear
B)
DNA and RNA
done
clear
C)
only RNA
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 317) Which of the following shows minimum number of chromosomes?
A)
n : 1
done
clear
B)
n : 2
done
clear
C)
n : 3
done
clear
D)
n : 4
done
clear
View Answer play_arrow
question_answer 318) Which of the following is formed in light reaction of photosynthesis?
A)
ATP
done
clear
B)
\[NADP{{H}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
All the above
done
clear
View Answer play_arrow
question_answer 319) In lysogenic phase which of the following takes place?
A)
Phage DNA integrated with host genome and does not multiplied
done
clear
B)
phage DNA does not integrate with host genome
done
clear
C)
Phage DNA integrates with host genome and bacterial cell does not lyse
done
clear
D)
Phage DNA integrated with host DNA and multiplied
done
clear
View Answer play_arrow
question_answer 320) Who proposed 'fluid mosaic model' of plasma membrane?
A)
Singer and Nicholson
done
clear
B)
Robertson
done
clear
C)
Benneson
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 321) The development of sporangium in Pteridium is a:
A)
leptosporangiate type
done
clear
B)
eusporangiate type
done
clear
C)
both and type
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 322) Respiratory roots (pneumatophores) are present in:
A)
mesophytes
done
clear
B)
halophytes
done
clear
C)
xerophytes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 323) The cell wall of fungi is formed by:
A)
chitin and mannose
done
clear
B)
cellulose
done
clear
C)
glucose
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 324) Which is present in vascular bundle of Pinus?
A)
Tracheids
done
clear
B)
Vessels
done
clear
C)
Companion cells
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 325) Growth of stem in diameter is due to:
A)
apical cell
done
clear
B)
lateral meristem
done
clear
C)
apical meristem
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow
question_answer 326) The plant group in which stomata opens in night is?
A)
Mesuphytes
done
clear
B)
Succulents
done
clear
C)
Hydrophytes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 327) The right equation for water potential is:
A)
\[\Psi ={{\Psi }_{p}}-(\Psi +{{\Psi }_{m}})\]
done
clear
B)
\[\Psi ={{\Psi }_{p}}+({{\Psi }_{s}}-{{\Psi }_{m}})\]
done
clear
C)
\[{{\Psi }_{w}}={{\Psi }_{s}}+{{\Psi }_{p}}\]
done
clear
D)
\[{{\Psi }_{w}}={{\Psi }_{p}}+{{\Psi }_{\pi }}+{{\Psi }_{m}}\]
done
clear
View Answer play_arrow
question_answer 328) The 'respiratory quotient' of fat is:
A)
0.7
done
clear
B)
1.5
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 329) The chloroplast in Ulothrix is:
A)
girdle shaped
done
clear
B)
stellate
done
clear
C)
reticulate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 330) The fruit of Solanaceae family is:
A)
Berry
done
clear
B)
Capsule
done
clear
C)
Pome
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 331) Gaseous plant hormone is:
A)
ethylene
done
clear
B)
auxin
done
clear
C)
gibberellin
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 332) Which of the following is not involve in symbiotic nitrogen fixation?
A)
Nostoc
done
clear
B)
Rhizobium
done
clear
C)
Oscillatoria
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 333) Alkaloid obtained from Papaver somniferum is:
A)
nicotine
done
clear
B)
cocaine
done
clear
C)
morphine
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 334) Transpiration rate will increase if:
A)
there is humid atmosphere and high wind
done
clear
B)
mere is humid atmosphere and low wind
done
clear
C)
there is high temperature and low wind
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 335) Which of the following test is used for AIDS?
A)
ELISA test
done
clear
B)
Western blot test
done
clear
C)
P.C.R.
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 336) Hormone which controls the stomatal movement is:
A)
GA
done
clear
B)
ABA
done
clear
C)
\[\text{IAA}\]
done
clear
D)
KA
done
clear
View Answer play_arrow
question_answer 337) The aquatic species of bryophytes are :
A)
Riccia fluitans and Ricciocarpus natans
done
clear
B)
fluitans and R. discolor
done
clear
C)
R fluitans and R. abbusieance
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 338) What is the reason for the presence of water drops on the leaves of plants in the morning?
A)
Secretion
done
clear
B)
Guttation
done
clear
C)
Tranpiration
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 339) In which colour the rate of photosynthesis is maximum?
A)
Violet
done
clear
B)
Green
done
clear
C)
Yellow
done
clear
D)
Red
done
clear
View Answer play_arrow
question_answer 340) The first carbon dioxide acceptor in Calvin cycle is:
A)
PEP carboxylase
done
clear
B)
RuBP carboxylase
done
clear
C)
RuBP oxygenase
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 341) The element which is found in chlorophyll molecule:
A)
B
done
clear
B)
Mg
done
clear
C)
Na
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 342) Yeast shows:
A)
ha p lob ion tic alternation of generation
done
clear
B)
diplobiontic alternation of generation
done
clear
C)
haplo-diplobiontic alternation of generation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 343) The longest gymnospermic plant is:
A)
Sequoia dendron
done
clear
B)
Pinns
done
clear
C)
Cycas
done
clear
D)
Cinchona
done
clear
View Answer play_arrow
question_answer 344) Krebs' cycle takes place in:
A)
matrix of mitochondria
done
clear
B)
cristae of mitochondria
done
clear
C)
membrane of mitochondria
done
clear
D)
cytoplasm
done
clear
View Answer play_arrow
question_answer 345) Family of Atropa belladonna is:
A)
Solanaceae
done
clear
B)
Malvaceae
done
clear
C)
Cruciferae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 346) Asafoetida is obtained from which part of the plant:
A)
Stem
done
clear
B)
Bud
done
clear
C)
Leaf
done
clear
D)
Root
done
clear
View Answer play_arrow
question_answer 347) False statement is:
A)
ribosome made up of ribo proteins
done
clear
B)
ribosome is single membrane bound organelle
done
clear
C)
membrane is absent in ribosome
done
clear
D)
ribosome consists of two sub units
done
clear
View Answer play_arrow
question_answer 348) Mycorrhiza is the relation of:
A)
algae and fungi
done
clear
B)
bryophyta and fungi
done
clear
C)
higher plants and fungi
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 349) Reserpine obtains from:
A)
Cinchona
done
clear
B)
Sarpgandha
done
clear
C)
Neem
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 350) From which part of the 'Cinchona' 'quenine' obtains?
A)
Bark of stem
done
clear
B)
Leaf
done
clear
C)
Root
done
clear
D)
flower
done
clear
View Answer play_arrow
question_answer 351) In a normal dicot leaf the phloem in the midrib faces:
A)
upper epidermis
done
clear
B)
lower epidermis
done
clear
C)
centre of vascular bundle
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 352) The embryo formed from ovule is known as:
A)
parthenogenesis
done
clear
B)
parthenocarpy
done
clear
C)
double fertilization
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 353) Phytochrome is similar to:
A)
carotene
done
clear
B)
xanthophyll
done
clear
C)
phycobilins
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 354) The cause of 'mad cow' disease in England was:
A)
screpia protein
done
clear
B)
bacteria
done
clear
C)
virus
done
clear
D)
mycoplasma
done
clear
View Answer play_arrow
question_answer 355) Active absorption of water is affected by:
A)
osmosis in cytoplasm
done
clear
B)
transpiration pull
done
clear
C)
osmatic tissue
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 356) The characteristics of family Malvaceae is:
A)
monadelphous
done
clear
B)
diadelphous
done
clear
C)
tetradelphous
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 357) Dark reaction takes place in:
A)
day
done
clear
B)
night
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 358) Gemma cups in Marchantia are present on:
A)
apical notch
done
clear
B)
ventral side of thallus
done
clear
C)
dorsal side of thallus
done
clear
D)
both dorsal and ventral side
done
clear
View Answer play_arrow
question_answer 359) Resolving power of compound microscope is:
A)
2.75 \[\mu m\]
done
clear
B)
27.5 \[\mu m\]
done
clear
C)
0.275 \[\mu m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 360) Cleistothecium forms in which of the following?
A)
Ascomycotina
done
clear
B)
Aspergillus
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 361) When two odd characters are present in a gene this is known as:
A)
heterogamous
done
clear
B)
dioecious
done
clear
C)
homogamous
done
clear
D)
monoecious
done
clear
View Answer play_arrow
question_answer 362) Which of the following is false statement?
A)
Bacteriophage attacking on E. coli is called T-Phage.
done
clear
B)
T-phage is tadpole in shape
done
clear
C)
\[\text{-}\]phage contains single thread
done
clear
D)
\[\text{-}\]phage contains two thread.
done
clear
View Answer play_arrow
question_answer 363) The zoospores of Oedogonium contains:
A)
aflagellate
done
clear
B)
ring of flagella
done
clear
C)
tetraflagellate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 364) The mycelium of Albugo is:
A)
haploid, septate, uninucleate
done
clear
B)
haploid, aseptate, multinucleate
done
clear
C)
diploid, septate, uninucleate
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 365) Avena curvature test' performed by:
A)
P. Scoog
done
clear
B)
F. W. Went
done
clear
C)
Odem
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 366) Who divide plants into four group on the basis of temperature?
A)
Warming
done
clear
B)
Odem
done
clear
C)
Raunkiaer
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 367) The main force for translocation is:
A)
cohesion force
done
clear
B)
endosmosis
done
clear
C)
electro-magnetic
done
clear
D)
osmotic power
done
clear
View Answer play_arrow
question_answer 368) The fertile layer of Agaricus is known as:
A)
hymenium
done
clear
B)
sub hymenium
done
clear
C)
trama
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 369) \[{{F}_{2}}\] ratio of monohybrid cross is:
A)
1 : 2
done
clear
B)
1 : 2 : 1
done
clear
C)
3 : 1
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 370) On selfing heterozygous, long, \[{{F}_{1}}\] generations plants both long and dwarf plants obtained in \[{{F}_{2}}\] generation this shows:
A)
law of dominance
done
clear
B)
law of segregation
done
clear
C)
law of independent assortment
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 371) Peristome of Funaria contains:
A)
4 teeths in single ring
done
clear
B)
32 teeths in two rings
done
clear
C)
16 teeths
done
clear
D)
18 teeths in two rings
done
clear
View Answer play_arrow
question_answer 372) Which of the following shows fermentation?
A)
Yeast
done
clear
B)
Gymnosperm
done
clear
C)
Angiosperm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 373) Which of the following is not xerophytic plant?
A)
Opuntia
done
clear
B)
Jujube
done
clear
C)
Caparis
done
clear
D)
Vallisneria
done
clear
View Answer play_arrow
question_answer 374) Plants who flowers single time in their life cycle is known as:
A)
monocarpic
done
clear
B)
holocarpic
done
clear
C)
polocarpic
done
clear
D)
eucarpic
done
clear
View Answer play_arrow
question_answer 375) Nucleic acid is absent in:
A)
nucleus
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
golgi body
done
clear
View Answer play_arrow
question_answer 376) The conditions in which photo respiration takes place are:
A)
high concentration of \[{{O}_{2}}\] and low \[C{{O}_{2}}\]
done
clear
B)
low light and high concentration of \[{{O}_{2}}\]
done
clear
C)
low temperature and high concentration of \[C{{O}_{2}}\]
done
clear
D)
low concentration of \[{{O}_{2}}\] and high \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 377) Which of the following is floral formula of Brassica campestris?
A)
\[\oplus \,{{K}_{4}}\,{{C}_{4}}\,{{A}_{4+\,2}}\,{{\underline{G}}_{(2)}}\,\]
done
clear
B)
\[\oplus \,{{K}_{4}}\,{{C}_{5}}\,{{A}_{10}}\,{{\underline{G}}_{(5)}}\,\]
done
clear
C)
\[%\,{{K}_{4}}\,{{C}_{2+3}}\,{{A}_{5}}\,{{\underline{G}}_{(2)}}\,\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 378) Root pressure is due to:
A)
passive absorption
done
clear
B)
active absorption
done
clear
C)
in creasement in transpiration
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 379) The last product of aerobic respiration is:
A)
starch and oxygen
done
clear
B)
water and energy
done
clear
C)
water, carbon in oxide and energy
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 380) Fermentation is found in:
A)
all fungus
done
clear
B)
all bacteria
done
clear
C)
some bacteria and some fungus
done
clear
D)
all micro-organism
done
clear
View Answer play_arrow
question_answer 381) Which electron carrier is found in both photo phosphorylation and oxidative phosphorylation?
A)
Oxygen
done
clear
B)
Carbon dioxide
done
clear
C)
Cytochrome
done
clear
D)
Water
done
clear
View Answer play_arrow
question_answer 382) The long chain fatty acids are oxidized by
A)
\[\beta \]-oxidation
done
clear
B)
\[\alpha \]-oxidation
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 383) Hill reaction is:
A)
absorption of oxygen
done
clear
B)
\[C{{O}_{2}}\] fixation
done
clear
C)
light reduction
done
clear
D)
photolysis of water
done
clear
View Answer play_arrow
question_answer 384) The country which produces maximum part of greenhouse gases in the world is:
A)
Russia
done
clear
B)
Germany
done
clear
C)
Brazil
done
clear
D)
America
done
clear
View Answer play_arrow
question_answer 385) The exarch primary xylem is found in:
A)
all roots
done
clear
B)
all leaves
done
clear
C)
monocot stem
done
clear
D)
dicot stem
done
clear
View Answer play_arrow
question_answer 386) Sieve tube is:
A)
without nucleus
done
clear
B)
multinucleate
done
clear
C)
uninucleate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 387) The monocot plant shows secondary growth is:
A)
Sugarcane
done
clear
B)
Coconut
done
clear
C)
Maize
done
clear
D)
Yucca
done
clear
View Answer play_arrow
question_answer 388) The function of smooth endoplasmic reticulum is:
A)
fat synthesis
done
clear
B)
protein synthesis
done
clear
C)
RNA synthesis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 389) Which of the following is related to Mycology?
A)
Dr. K.C, Mehta
done
clear
B)
Dr. S.R. Kashyap
done
clear
C)
Dr. P. Maheshwari
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 390) Which of the following is parasitic nature?
A)
Laminaria
done
clear
B)
Cephaleuros
done
clear
C)
Chara
done
clear
D)
Sytonema
done
clear
View Answer play_arrow
question_answer 391) In photosynthesis oxygen is evolved From:
A)
water
done
clear
B)
carbon dioxide
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 392) Synapsis takes place in:
A)
leptotene phase
done
clear
B)
zygotene phase
done
clear
C)
pachytene phase
done
clear
D)
diplotcne phase
done
clear
View Answer play_arrow
question_answer 393) Famous famine of Bengal in 1942-43 was due to destruction of Rice crop by:
A)
Helminthosporium
done
clear
B)
Rhizopus
done
clear
C)
Puccinia
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 394) The change of starch into carbonic acid is related with:
A)
closing of stomata
done
clear
B)
opening of stomata
done
clear
C)
stimulation of stomata
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 395) \[{{C}_{4}}-\]plants is also known as:
A)
Calvin type
done
clear
B)
Hatch and Slack type
done
clear
C)
Emmersion type
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 396) DNA is made up of:
A)
nucleotides
done
clear
B)
nucleosides
done
clear
C)
polypeptides
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 397) Which of the following is produced by the degradation of carbohydrate an aerobically?
A)
Fructose \[+{{H}_{2}}O\]
done
clear
B)
\[C{{O}_{2}}+{{H}_{2}}O\]
done
clear
C)
Glucose \[+{{H}_{2}}O\]
done
clear
D)
Alcohol \[+C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 398) Tylosis are found in:
A)
xylem vessels
done
clear
B)
phloem tracheids
done
clear
C)
cambium
done
clear
D)
none of the above
done
clear
View Answer play_arrow