Reproduction, Control and Coordination
All the organisms reproduce to continue their existence on the earth. The production of new organism from the existing organisms of the same species is called reproduction. It is a necessary process to maintain the life on the earth. There are several ways through which animals can produce offspring. The two main methods of reproduction are sexual and asexual.
Sexual Reproduction
The production of new organism with the use of their sex gametes is called sexual reproduction. This type of reproduction requires two parents who donate genes to the young one, resulting in offspring with a mix of inherited genes. Humans, animals and many other organisms reproduce by this method. Many flowering plants also reproduce by this method.
Asexual Reproduction
The production of new organism without the involvement of sex gametes is called asexual reproduction. In this type of reproduction, only a single parent is required.
Types of Asexual reproduction:
Fragmentation
In fragmentation, parent breaks different fragments, which eventually forms new individuals. For example, spirogyra.
Regeneration
In regeneration, when an animal that is capable of regeneration loses a body part, it can grow a replacement part. If the lost body part contains enough genetic information from the parent, it can regenerate into an entirely new organism. For example, sea stars, flatworms, etc.
Budding
In budding, a bulb- like projection or outgrowth arises from the parent body known as bud which detaches and forms a new organism. These buds develop into tiny individuals and when get fully mature.
Vegetative propagation
In this type of reproduction, any vegetative part of the plant body like leaf, stem or root develops into a complete new plant. For example, leaf in bryophyllum, stem in rose, bulb in onion, etc.
Spore formation
In this mode of reproduction, the organism breaks up into a number of pieces or spores, each of which eventually develops into an organism. Spore formation is a mode of reproduction resembling multiple fission. For example. Ferns, Mosses, Rhizopus, etc.
Sexual Reproduction in Flowering Plants
In sexual reproduction, the male cell produced by the male part of the flower and female cell produced by the female part of the flower fuses together. The male and female cells are called gametes. The fusion of male and female gametes is known as fertilization and leads to the formation of single cell, called zygote. The zygote divides repeatedly and gives rise to a new individual.
Structure of a flower:
The flower consists of four whorls. The outermost whorl consists of sepals. Then next is petals. Then after that comes stamens and at the centre is the female whorl, called pistil. The pistil can consist of one or many carpels. The carpel has a stalklike style with a sticky tip called the stigma and swollen base called ovary. Inside the ovary, there exists egg like ovules.
All flowers do not have all the four whorls. Flowers having all
more...
 The carbon atom chain can be cyclic or closed rings, sheets and even three-dimensional lattices.
The carbon atom chain can be cyclic or closed rings, sheets and even three-dimensional lattices.
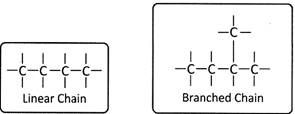
 When there are more than three carbon atoms, compounds can be branched. The branched chains provide different structure to the parent alkane and are named differently. For example, in pentane \[({{C}_{5}}{{H}_{12}})\] there exist a straight chain and branched chain called Iso-pentane. Carbon can thus form large number of more...
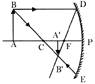
When there are more than three carbon atoms, compounds can be branched. The branched chains provide different structure to the parent alkane and are named differently. For example, in pentane \[({{C}_{5}}{{H}_{12}})\] there exist a straight chain and branched chain called Iso-pentane. Carbon can thus form large number of more... 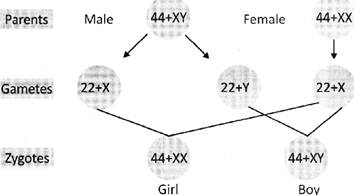 Sex determination of a child
Evolution
Evolution is the series of gradual changes that take place over millions of years. It is the change in the genetic material of a population of organisms from one generation to another. Genes are the basis of evolution that passes from one generation to another and thus produces an organism's inherited traits. The inherited traits vary within organisms.
The mechanisms that determine which variant will become more common or rare in a population are natural selection and genetic drift. Natural selection is a process that causes helpful traits to become more common in a population and harmful traits to become rarer. This happens because individuals with useful traits are more likely to reproduce. This clearly indicates that more individuals in the next generation will inherit these traits. Adaptations occur through a combination of successive, small, random changes in traits over many generations and natural selection of the variants best-suited for their environment. Genetic drift is an independent process that produces random changes in the frequency of traits in a population. Genetic drift results from the disappearance of particular genes as individuals die more...
Sex determination of a child
Evolution
Evolution is the series of gradual changes that take place over millions of years. It is the change in the genetic material of a population of organisms from one generation to another. Genes are the basis of evolution that passes from one generation to another and thus produces an organism's inherited traits. The inherited traits vary within organisms.
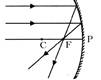
The mechanisms that determine which variant will become more common or rare in a population are natural selection and genetic drift. Natural selection is a process that causes helpful traits to become more common in a population and harmful traits to become rarer. This happens because individuals with useful traits are more likely to reproduce. This clearly indicates that more individuals in the next generation will inherit these traits. Adaptations occur through a combination of successive, small, random changes in traits over many generations and natural selection of the variants best-suited for their environment. Genetic drift is an independent process that produces random changes in the frequency of traits in a population. Genetic drift results from the disappearance of particular genes as individuals die more...  Rules for obtaining images formed by concave mirrors
Rules for obtaining images formed by concave mirrors