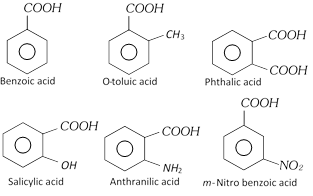
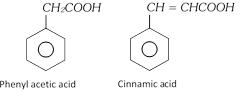
 Aromatic acid containing –COOH group in the side chain, they are considered as aryl substituted aliphatic acid.
Examples
Aromatic acid containing –COOH group in the side chain, they are considered as aryl substituted aliphatic acid.
Examples
 Benzoic Acid
(1) Methods of Preparation
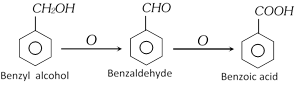
(i) From oxidation of Benzyl alcohol [Laboratory method]
Benzoic Acid
(1) Methods of Preparation
(i) From oxidation of Benzyl alcohol [Laboratory method]
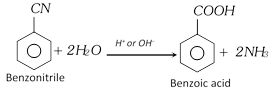
 (ii) From hydrolysis of nitriles or cyanides
(ii) From hydrolysis of nitriles or cyanides
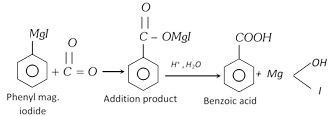
 (iii) From Grignard reagent
(iii) From Grignard reagent
 (iv) By hydrolysis of esters
\[\underset{\text{Methyl benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}COOC{{H}_{3}}}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}orO{{H}^{-}}}\underset{\text{Benzoic acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,+\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,\]
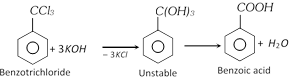
(v) From trihalogen derivatives of hydrocarbons
(iv) By hydrolysis of esters
\[\underset{\text{Methyl benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}COOC{{H}_{3}}}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}orO{{H}^{-}}}\underset{\text{Benzoic acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,+\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,\]
(v) From trihalogen derivatives of hydrocarbons
 (vi) From benzene
(vi) From benzene
 (vii) From Toluene
(vii) From Toluene
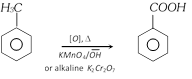
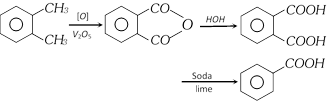 (ix) From naphthalene [Industrial method]
(ix) From naphthalene [Industrial method]
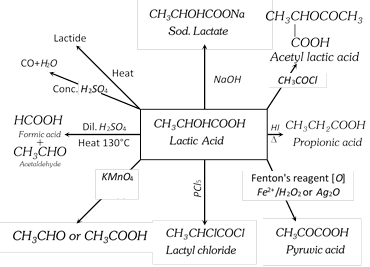 (4) Uses : It is used in medicine as calcium and iron lactates, as mordant in dyeing, as acidulant in beverages and candies, as a solvent (ethyl and butyl lactates) for cellulose nitrate.
Tartaric Acid. Or \[\alpha ,\,\,\alpha '-\]Dihydroxy succinic acid or 2, 3-Dihydroxy-Butane-1,4-Dioic acid
\[\underset{HO-CH-COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,H-COOH}}\,\]
It is found as free or potassium salt in grapes, tamarind, and berries.
(1) Methods of Preparation
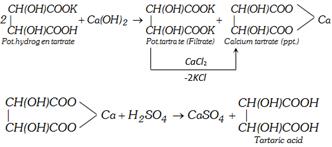
(i) Argol which separates as a crust during fermentation of grape juice is impure potassium hydrogen tartrate. Argol is boiled with limewater. Calcium tartrate is precipitated which is filtered. The solution contains potassium tartrate which is also precipitated by addition of \[CaC{{l}_{2}}\]. The calcium salt is then decomposed with calculated quantity of dilute \[{{H}_{2}}S{{O}_{4}}\]. The precipitate \[(CaS{{O}_{4}})\] is filtered and the filtrate on concentration gives the crystals of tartaric acid.
(4) Uses : It is used in medicine as calcium and iron lactates, as mordant in dyeing, as acidulant in beverages and candies, as a solvent (ethyl and butyl lactates) for cellulose nitrate.
Tartaric Acid. Or \[\alpha ,\,\,\alpha '-\]Dihydroxy succinic acid or 2, 3-Dihydroxy-Butane-1,4-Dioic acid
\[\underset{HO-CH-COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,H-COOH}}\,\]
It is found as free or potassium salt in grapes, tamarind, and berries.
(1) Methods of Preparation
(i) Argol which separates as a crust during fermentation of grape juice is impure potassium hydrogen tartrate. Argol is boiled with limewater. Calcium tartrate is precipitated which is filtered. The solution contains potassium tartrate which is also precipitated by addition of \[CaC{{l}_{2}}\]. The calcium salt is then decomposed with calculated quantity of dilute \[{{H}_{2}}S{{O}_{4}}\]. The precipitate \[(CaS{{O}_{4}})\] is filtered and the filtrate on concentration gives the crystals of tartaric acid.
 (ii) Synthetic method
\[C+{{H}_{2}}\underset{arc}{\mathop{\xrightarrow{\text{Electric}}}}\,\underset{\text{Acetylene}}{\mathop{CH\equiv CH}}\,\underset{{Pd}/{BaS{{O}_{4}}}\;}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{B{{r}_{2}}}\]
\[\underset{\text{Ethylene bromide}}{\mathop{{{(C{{H}_{2}}Br)}_{2}}}}\,\xrightarrow{2KCN}\underset{C{{H}_{2}}CN}{\overset{C{{H}_{2}}CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Succinic acid}}{\mathop{\underset{C{{H}_{2}}C{{O}_{2}}H}{\overset{C{{H}_{2}}C{{O}_{2}}H}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
\[\underset{B{{r}_{2}}}{\mathop{\xrightarrow{\operatorname{Re}\text{d}\,P}}}\,\underset{\begin{smallmatrix} \alpha \text{,}\alpha \text{ }\!\!'\!\!\text{ -Dibromo succinic} \\ \text{ acid} \end{smallmatrix}}{\mathop{\underset{CHBrCOOH}{\overset{CHBrCOOH} {\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\] \[\xrightarrow{AgOH}\underset{\text{Tartaric acid}}{\mathop{\underset{CHOHCOOH}{\overset{CHOHCOOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iii) From glyoxal cyanohydrin :
\[\underset{\text{Glyoxal}}{\mathop{\underset{CHO}{\overset{CHO}{\mathop{|\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{HCN}\underset{\text{Glyoxal cyanohydrin}}{\mathop{\underset{CH(OH)CN}{\overset{CH(OH)CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Tartaric acid}}{\mathop{\underset{CH(OH)COOH}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
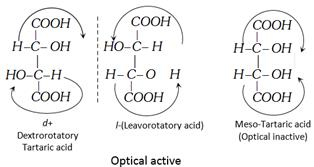
(2) Physical Properties : It is a colourless crystalline compound. It is soluble in water and alcohol but insoluble in ether. It contains two asymmetric carbon atoms and thus shows optical isomerism (four forms). Natural tartaric acid is the dextro variety. It contains two secondary alcoholic groups and two carboxylic groups.
Optical Isomerism in tartaric acid
(ii) Synthetic method
\[C+{{H}_{2}}\underset{arc}{\mathop{\xrightarrow{\text{Electric}}}}\,\underset{\text{Acetylene}}{\mathop{CH\equiv CH}}\,\underset{{Pd}/{BaS{{O}_{4}}}\;}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{B{{r}_{2}}}\]
\[\underset{\text{Ethylene bromide}}{\mathop{{{(C{{H}_{2}}Br)}_{2}}}}\,\xrightarrow{2KCN}\underset{C{{H}_{2}}CN}{\overset{C{{H}_{2}}CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Succinic acid}}{\mathop{\underset{C{{H}_{2}}C{{O}_{2}}H}{\overset{C{{H}_{2}}C{{O}_{2}}H}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
\[\underset{B{{r}_{2}}}{\mathop{\xrightarrow{\operatorname{Re}\text{d}\,P}}}\,\underset{\begin{smallmatrix} \alpha \text{,}\alpha \text{ }\!\!'\!\!\text{ -Dibromo succinic} \\ \text{ acid} \end{smallmatrix}}{\mathop{\underset{CHBrCOOH}{\overset{CHBrCOOH} {\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\] \[\xrightarrow{AgOH}\underset{\text{Tartaric acid}}{\mathop{\underset{CHOHCOOH}{\overset{CHOHCOOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iii) From glyoxal cyanohydrin :
\[\underset{\text{Glyoxal}}{\mathop{\underset{CHO}{\overset{CHO}{\mathop{|\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{HCN}\underset{\text{Glyoxal cyanohydrin}}{\mathop{\underset{CH(OH)CN}{\overset{CH(OH)CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Tartaric acid}}{\mathop{\underset{CH(OH)COOH}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Physical Properties : It is a colourless crystalline compound. It is soluble in water and alcohol but insoluble in ether. It contains two asymmetric carbon atoms and thus shows optical isomerism (four forms). Natural tartaric acid is the dextro variety. It contains two secondary alcoholic groups and two carboxylic groups.
Optical Isomerism in tartaric acid
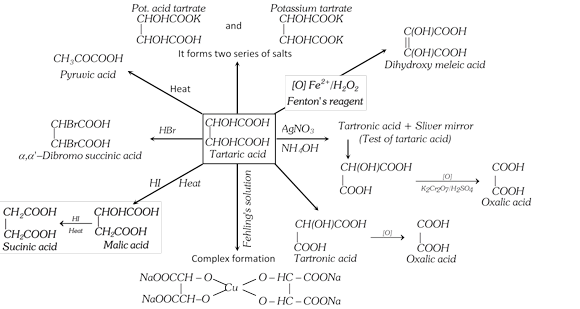 (4) Uses : It is used in carbonated beverages more...
(4) Uses : It is used in carbonated beverages more... | Name of acids | Source | Molecular formula |
| Palmitic acid | Palm oil | \[C{{H}_{3}}{{(C{{H}_{2}})}_{14}}COOH\] |
| Stearic acid | Stear (meaning tallow) | \[C{{H}_{3}}{{(C{{H}_{2}})}_{16}}COOH\] |
| Oleic acid | Olive oil. | \[C{{H}_{3}}{{(C{{H}_{2}})}_{7}}CH=CH{{(C{{H}_{2}})}_{7}}COOH\] |
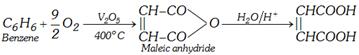 (ii) From malic acid :
(ii) From malic acid :
 \[\underset{\text{boil}}{\mathop{\xrightarrow{NaOH}}}\,\underset{\text{Sodium salt}}{\mathop{\underset{CH-COONa}{\overset{CH-COONa}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}^{+}}}/{{{H}_{2}}O}\;}\underset{\text{Maleic acid}}{\mathop{\underset{CH-COOH}{\overset{CH-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Methods of Preparation of Fumaric Acid
(i) From maleic acid :
\[\underset{\text{Maleic acid}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{boil}}{\mathop{\xrightarrow{HCl}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
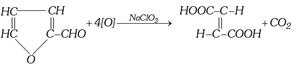
(ii) By oxidation of furfural with sodium chlorate
\[\underset{\text{boil}}{\mathop{\xrightarrow{NaOH}}}\,\underset{\text{Sodium salt}}{\mathop{\underset{CH-COONa}{\overset{CH-COONa}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}^{+}}}/{{{H}_{2}}O}\;}\underset{\text{Maleic acid}}{\mathop{\underset{CH-COOH}{\overset{CH-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Methods of Preparation of Fumaric Acid
(i) From maleic acid :
\[\underset{\text{Maleic acid}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{boil}}{\mathop{\xrightarrow{HCl}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
(ii) By oxidation of furfural with sodium chlorate
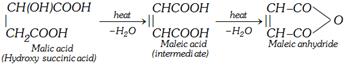
 (iii) By heating malic acid at about \[150{}^\circ C\] for long time
\[\underset{\text{Malic acid}}{\mathop{\underset{C{{H}_{2}}COOH\,\,\,\,\,\,\,\,}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{150{}^\circ C,\,\,-{{H}_{2}}O}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
(iv) By heating bromosuccinic acid with alcoholic potash : By heating bromosuccinic acid with alcoholic potash.
\[\underset{CH.(Br)COOH}{\overset{C{{H}_{2}}COOH\,\,\,\,\,\,}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{\text{Alc}\text{. }KOH}\underset{\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H\,\,\,\,\,\,\,\,\,}{\mathop{\,|\,|}}}\,+KBr+{{H}_{2}}O\]
(3) Physical Properties
(i) Both are colourless crystalline solids. Both are soluble in water.
(ii) The melting point of maleic acid \[(130.5{}^\circ C)\] is lower than the melting point of fumaric acid \[(287{}^\circ C)\].
(4) Chemical Properties
Chemically, both the acids give the reactions of alkenes and dibasic acids except that the maleic acid on heating forms an anhydride while fumaric acid does not give anhydride.
\[\underset{\text{Maleic acid}}{\mathop{\underset{CHCOOH}{\overset{CHCOOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{\text{hea}\text{t}}\underset{\text{Maleic anhydride}}{\mathop{\underset{CHCO}{\overset{CHCO}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,\ \ \ \ \ O}}\,+{{H}_{2}}O\]
Both form succinic acid on reduction with sodium amalgam. They undergo addition reactions with bromine, hydrobromic acid, water, etc. and form salts, esters and acid chlorides as usual. With alkaline \[KMn{{O}_{4}}\] solution, they get oxidised to tartaric acid.
\[\underset{\begin{smallmatrix} \text{Tartaric acid} \\ \text{ (Meso)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-OH}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,} {\mathop{|\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{.}KMn{{O}_{4}}}}}\,\underset{\begin{smallmatrix} \text{Maleic acid} \\ \text{ (}Cis\text{)} \end{smallmatrix}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{(Racemic mixture)}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{Br-\underset{|}{\mathop{C}}\,-H}}\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|\,}}}\,}}\,\]
\[\underset{\begin{smallmatrix} \text{ Tartaric acid} \\ \text{(Racemic mixture)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,-H\,\,\,\,\,}}\,\,\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,}{\mathop{|\,\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{. }KMn{{O}_{4}}}}}\,\underset{\text{Fumaric acid (}Trans\text{)}}{\mathop{\underset{HOOC-C-H\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\mathop{|\,|}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{((Meso)}}{\mathop{\underset{\,\underset{\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-Br}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|}}}\,}}\,\]
(iii) By heating malic acid at about \[150{}^\circ C\] for long time
\[\underset{\text{Malic acid}}{\mathop{\underset{C{{H}_{2}}COOH\,\,\,\,\,\,\,\,}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{150{}^\circ C,\,\,-{{H}_{2}}O}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
(iv) By heating bromosuccinic acid with alcoholic potash : By heating bromosuccinic acid with alcoholic potash.
\[\underset{CH.(Br)COOH}{\overset{C{{H}_{2}}COOH\,\,\,\,\,\,}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{\text{Alc}\text{. }KOH}\underset{\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H\,\,\,\,\,\,\,\,\,}{\mathop{\,|\,|}}}\,+KBr+{{H}_{2}}O\]
(3) Physical Properties
(i) Both are colourless crystalline solids. Both are soluble in water.
(ii) The melting point of maleic acid \[(130.5{}^\circ C)\] is lower than the melting point of fumaric acid \[(287{}^\circ C)\].
(4) Chemical Properties
Chemically, both the acids give the reactions of alkenes and dibasic acids except that the maleic acid on heating forms an anhydride while fumaric acid does not give anhydride.
\[\underset{\text{Maleic acid}}{\mathop{\underset{CHCOOH}{\overset{CHCOOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{\text{hea}\text{t}}\underset{\text{Maleic anhydride}}{\mathop{\underset{CHCO}{\overset{CHCO}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,\ \ \ \ \ O}}\,+{{H}_{2}}O\]
Both form succinic acid on reduction with sodium amalgam. They undergo addition reactions with bromine, hydrobromic acid, water, etc. and form salts, esters and acid chlorides as usual. With alkaline \[KMn{{O}_{4}}\] solution, they get oxidised to tartaric acid.
\[\underset{\begin{smallmatrix} \text{Tartaric acid} \\ \text{ (Meso)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-OH}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,} {\mathop{|\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{.}KMn{{O}_{4}}}}}\,\underset{\begin{smallmatrix} \text{Maleic acid} \\ \text{ (}Cis\text{)} \end{smallmatrix}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{(Racemic mixture)}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{Br-\underset{|}{\mathop{C}}\,-H}}\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|\,}}}\,}}\,\]
\[\underset{\begin{smallmatrix} \text{ Tartaric acid} \\ \text{(Racemic mixture)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,-H\,\,\,\,\,}}\,\,\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,}{\mathop{|\,\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{. }KMn{{O}_{4}}}}}\,\underset{\text{Fumaric acid (}Trans\text{)}}{\mathop{\underset{HOOC-C-H\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\mathop{|\,|}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{((Meso)}}{\mathop{\underset{\,\underset{\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-Br}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|}}}\,}}\,\]
| Formula | Common name | IUPAC name |
| \[HOOCCOOH\] | Oxalic acid | Ethanedioic acid |
| \[HOOCC{{H}_{2}}COOH\] | Malonic acid | 1-3 Propanedioic acid |
| \[HOOCC{{H}_{2}}C{{H}_{2}}COOH\] | Succinic acid | 1,4-Butanedioic acid |
| \[HOOC{{(C{{H}_{2}})}_{3}}COOH\] | Glutaric acid | 1,5-Pentanedioic acid |
| \[HOOC{{(C{{H}_{2}})}_{4}}COOH\] | Adipic acid | 1,6-Hexanedioic acid |
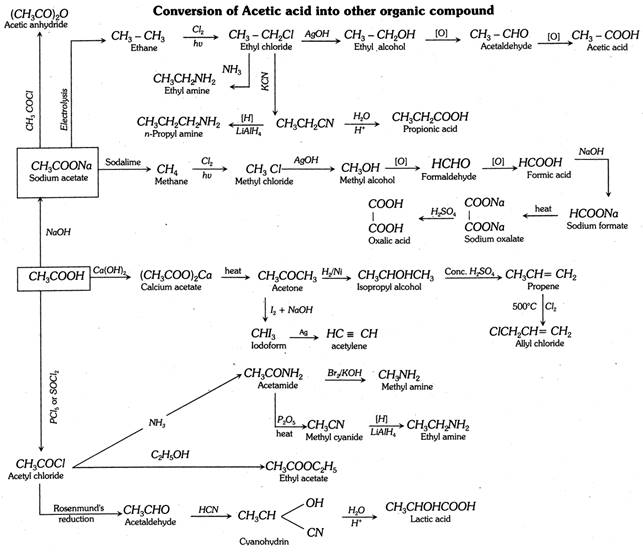
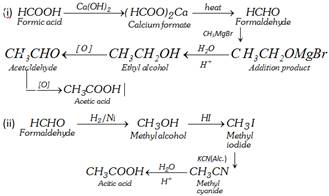 Arndt-Eistert homologation :
This is a convenient method of converting an acid, RCOOH to \[RC{{H}_{2}}COOH\].
(2) Descent of series :
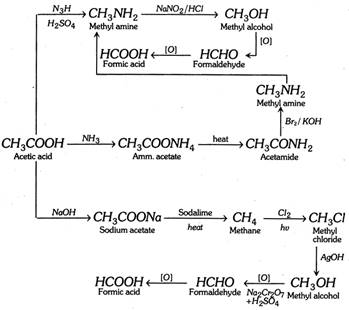
Conversion of acetic acid into formic acid.
Arndt-Eistert homologation :
This is a convenient method of converting an acid, RCOOH to \[RC{{H}_{2}}COOH\].
(2) Descent of series :
Conversion of acetic acid into formic acid.
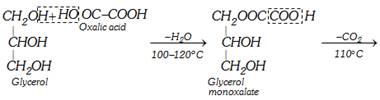 \[\underset{\begin{smallmatrix} \text{Glycerol } \\ \text{monoformate} \end{smallmatrix}}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,\,} {\overset{C{{H}_{2}}OOCH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{(COOH)}_{2}}2{{H}_{2}}O}\underset{\text{Formic acid}}{\mathop{HCOOH}}\,+\underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,}}}\,}}\,\]
The following procedure is applied for obtaining anhydrous formic acid.
\[2HCOOH+PbC{{O}_{3}}\to \underset{\text{Lead formate}}{\mathop{{{(HCOO)}_{2}}Pb}}\,+C{{O}_{2}}+{{H}_{2}}O\];
\[{{(HCOO)}_{2}}Pb+{{H}_{2}}S\to \underset{\text{ppt}\text{.}}{\mathop{PbS}}\,+\underset{\text{Formic acid}}{\mathop{2HCOOH}}\,\]
(iv) Industrial preparation : Formic acid is prepared on industrial scale by heating sodium hydroxide with carbon monoxide at \[210{}^\circ C\] under a pressure of about 10 atmospheres.
\[CO+NaOH\underset{{{210}^{o}}C,\,10\,\text{atm}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\Delta \,\,\,\,\,\,\,\,}}}\,\underset{\text{Sodium formate}}{\mathop{HCOONa}}\,\]
Sodium formate thus formed is distilled with sodium hydrogen sulphate, when anhydrous formic acid distils over.
\[HCOONa+NaHS{{O}_{4}}\to HCOOH+N{{a}_{2}}S{{O}_{4}}\]
(2) Physical properties
(i) It is a colourless pungent smelling liquid.
(ii) It melts at \[8.4{}^\circ C\] and boils at \[100.5{}^\circ C\].
(iii) It is miscible with water, alcohol and ether. It forms azeotropic mixture with water.
(iv) It is strongly corrosive and cause blisters on skin.
(v) It exists in aqueous solution as a dimer involving hydrogen bonding.
(3) Uses : Formic acid is used.
(i) In the laboratory for preparation of carbon monoxide.
(ii) In the preservation of fruits.
(iii) In textile dyeing and finishing.
(iv) In leather tanning.
(v) As coagulating agent for rubber latex.
(vi) As an antiseptic and in the treatment of gout.
(vii) In the manufacture of plastics, water proofing compounds.
(viii) In electroplating to give proper deposit of metals.
(ix) In the preparation of nickel formate which is used as a catalyst in the hydrogenation of oils.
(x) As a reducing agent.
(xi) In the manufacture of oxalic acid.
Acetic Acid (Ethanoic Acid) \[(C{{H}_{3}}COOH)\]
Acetic acid is the oldest known fatty acid. It is the chief constituent of vinegar and hence its name (Latin acetum = vinegar)
(1) Preparation
(i) By oxidation of acetaldehyde (Laboratory-preparation)
\[C{{H}_{3}}CHO\underset{{{H}_{2}}S{{O}_{4}}(O)}{\mathop{\xrightarrow{N{{a}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}COOH\]
(ii) By hydrolysis of methyl cyanide with acid
\[C{{H}_{3}}CN+2{{H}_{2}}O\xrightarrow{HCl}C{{H}_{3}}COOH+N{{H}_{3}}\]
(iii) By Grignard reagent
\[C{{H}_{3}}MgBr+C{{O}_{2}}\to C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OMgBr\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\] \[\left( C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH \right)\]
(iv) By hydrolysis of acetyl chloride, acetic anhydride or acetamide and ester
(a) \[\underset{\text{Ester}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(conc}\text{.)}}\] \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\]
(b) \[\underset{\text{acetylchloride}}{\mathop{C{{H}_{3}}COCl+{{H}_{2}}O}}\,\xrightarrow{\text{dil}\text{.}\,HCl}C{{H}_{3}}COOH+HCl\]
(c) \[{{\left( C{{H}_{3}}CO \right)}_{2}}O+{{H}_{2}}O\xrightarrow{\text{dil}\text{.}\,HCl}2C{{H}_{3}}COOH\]
(v) Manufacture of acetic acid
(a) From ethyl alcohol (Quick vinegar process) : Vinegar is 6-10% aqueous solution of acetic acid. It is obtained by fermentation of liquors containing 12 to 15% ethyl alcohol. Fermentation is done by Bacterium Mycoderma aceti in presence of air at 30-35°C. The process is termed acetous fermentation.
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+{{O}_{2}}\underset{\text{Bacter}\text{ia}}{\mathop{\xrightarrow{\text{Mycoderma aceti}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+{{H}_{2}}O\] more...
\[\underset{\begin{smallmatrix} \text{Glycerol } \\ \text{monoformate} \end{smallmatrix}}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,\,} {\overset{C{{H}_{2}}OOCH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{(COOH)}_{2}}2{{H}_{2}}O}\underset{\text{Formic acid}}{\mathop{HCOOH}}\,+\underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,}}}\,}}\,\]
The following procedure is applied for obtaining anhydrous formic acid.
\[2HCOOH+PbC{{O}_{3}}\to \underset{\text{Lead formate}}{\mathop{{{(HCOO)}_{2}}Pb}}\,+C{{O}_{2}}+{{H}_{2}}O\];
\[{{(HCOO)}_{2}}Pb+{{H}_{2}}S\to \underset{\text{ppt}\text{.}}{\mathop{PbS}}\,+\underset{\text{Formic acid}}{\mathop{2HCOOH}}\,\]
(iv) Industrial preparation : Formic acid is prepared on industrial scale by heating sodium hydroxide with carbon monoxide at \[210{}^\circ C\] under a pressure of about 10 atmospheres.
\[CO+NaOH\underset{{{210}^{o}}C,\,10\,\text{atm}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\Delta \,\,\,\,\,\,\,\,}}}\,\underset{\text{Sodium formate}}{\mathop{HCOONa}}\,\]
Sodium formate thus formed is distilled with sodium hydrogen sulphate, when anhydrous formic acid distils over.
\[HCOONa+NaHS{{O}_{4}}\to HCOOH+N{{a}_{2}}S{{O}_{4}}\]
(2) Physical properties
(i) It is a colourless pungent smelling liquid.
(ii) It melts at \[8.4{}^\circ C\] and boils at \[100.5{}^\circ C\].
(iii) It is miscible with water, alcohol and ether. It forms azeotropic mixture with water.
(iv) It is strongly corrosive and cause blisters on skin.
(v) It exists in aqueous solution as a dimer involving hydrogen bonding.
(3) Uses : Formic acid is used.
(i) In the laboratory for preparation of carbon monoxide.
(ii) In the preservation of fruits.
(iii) In textile dyeing and finishing.
(iv) In leather tanning.
(v) As coagulating agent for rubber latex.
(vi) As an antiseptic and in the treatment of gout.
(vii) In the manufacture of plastics, water proofing compounds.
(viii) In electroplating to give proper deposit of metals.
(ix) In the preparation of nickel formate which is used as a catalyst in the hydrogenation of oils.
(x) As a reducing agent.
(xi) In the manufacture of oxalic acid.
Acetic Acid (Ethanoic Acid) \[(C{{H}_{3}}COOH)\]
Acetic acid is the oldest known fatty acid. It is the chief constituent of vinegar and hence its name (Latin acetum = vinegar)
(1) Preparation
(i) By oxidation of acetaldehyde (Laboratory-preparation)
\[C{{H}_{3}}CHO\underset{{{H}_{2}}S{{O}_{4}}(O)}{\mathop{\xrightarrow{N{{a}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}COOH\]
(ii) By hydrolysis of methyl cyanide with acid
\[C{{H}_{3}}CN+2{{H}_{2}}O\xrightarrow{HCl}C{{H}_{3}}COOH+N{{H}_{3}}\]
(iii) By Grignard reagent
\[C{{H}_{3}}MgBr+C{{O}_{2}}\to C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OMgBr\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\] \[\left( C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH \right)\]
(iv) By hydrolysis of acetyl chloride, acetic anhydride or acetamide and ester
(a) \[\underset{\text{Ester}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(conc}\text{.)}}\] \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\]
(b) \[\underset{\text{acetylchloride}}{\mathop{C{{H}_{3}}COCl+{{H}_{2}}O}}\,\xrightarrow{\text{dil}\text{.}\,HCl}C{{H}_{3}}COOH+HCl\]
(c) \[{{\left( C{{H}_{3}}CO \right)}_{2}}O+{{H}_{2}}O\xrightarrow{\text{dil}\text{.}\,HCl}2C{{H}_{3}}COOH\]
(v) Manufacture of acetic acid
(a) From ethyl alcohol (Quick vinegar process) : Vinegar is 6-10% aqueous solution of acetic acid. It is obtained by fermentation of liquors containing 12 to 15% ethyl alcohol. Fermentation is done by Bacterium Mycoderma aceti in presence of air at 30-35°C. The process is termed acetous fermentation.
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+{{O}_{2}}\underset{\text{Bacter}\text{ia}}{\mathop{\xrightarrow{\text{Mycoderma aceti}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+{{H}_{2}}O\] more... 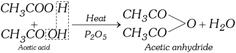 (vi) Reaction with organo-metallic reagents
\[R'C{{H}_{2}}MgBr+RCOOH\xrightarrow{\text{ether}}\underset{\text{Alkane}}{\mathop{R'C{{H}_{3}}}}\,+RCOOMgBr\]
(3) Reaction involving carbonyl \[(>C=O)\] group:
Reduction : \[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{LiAl{{H}_{4}}}R-C{{H}_{2}}-OH\]
Carboxylic acid are difficult to reduce either by catalytic hydrogenation or \[{Na}/{{{C}_{2}}{{H}_{5}}OH}\;\]
(4) Reaction involving attack of carboxylic group \[(-COOH)\]
(i) Decarboxylation : \[R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{(-C{{O}_{2}})}R-H\]
When anhydrous alkali salt of fatty acid is heated with sodalime then :
\[\underset{\text{Sodium salt}}{\mathop{RCOONa}}\,+NaOH\underset{\text{heat}}{\mathop{\xrightarrow{CaO}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\]
(vi) Reaction with organo-metallic reagents
\[R'C{{H}_{2}}MgBr+RCOOH\xrightarrow{\text{ether}}\underset{\text{Alkane}}{\mathop{R'C{{H}_{3}}}}\,+RCOOMgBr\]
(3) Reaction involving carbonyl \[(>C=O)\] group:
Reduction : \[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{LiAl{{H}_{4}}}R-C{{H}_{2}}-OH\]
Carboxylic acid are difficult to reduce either by catalytic hydrogenation or \[{Na}/{{{C}_{2}}{{H}_{5}}OH}\;\]
(4) Reaction involving attack of carboxylic group \[(-COOH)\]
(i) Decarboxylation : \[R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{(-C{{O}_{2}})}R-H\]
When anhydrous alkali salt of fatty acid is heated with sodalime then :
\[\underset{\text{Sodium salt}}{\mathop{RCOONa}}\,+NaOH\underset{\text{heat}}{\mathop{\xrightarrow{CaO}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\]
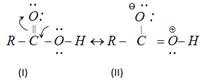 (ii) Due to electron deficiency on oxygen atom of the hydroxyl group (Structure II), their is a displacement of electron pair of O?H bond toward the oxygen atom. This facilitate the release of hydrogen as proton (H+).
(ii) Due to electron deficiency on oxygen atom of the hydroxyl group (Structure II), their is a displacement of electron pair of O?H bond toward the oxygen atom. This facilitate the release of hydrogen as proton (H+).
 (ii) An electron releasing substituent (+ I effect) stabilizes negative charge on the anion resulting in the decrease of stability and thus decreased the acidity of acid.
Electron with drawing nature of halogen : F > Cl > Br > I
Thus, the acidic strength decreases in the order :
\[FC{{H}_{2}}COOH>ClC{{H}_{2}}COOH>BrC{{H}_{2}}COOH>IC{{H}_{2}}COOH\]
similarly :
\[CC{{l}_{3}}COOH>CHC{{l}_{2}}COOH>C{{H}_{2}}ClCOOH>C{{H}_{3}}COOH\]
(iii) Inductive effect is stronger at \[\alpha -\]position than \[\beta -\]position similarly at \[\beta -\]position it is more stronger than at \[\gamma -\]position
Example:
\[C{{H}_{3}}-C{{H}_{2}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-COOH>C{{H}_{3}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-COOH\] \[>\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-C{{H}_{2}}-C{{H}_{2}}-COOH\]
(iv) Relative acid strength in different compounds
\[RCOOH>HOH>ROH>HC\equiv CH>N{{H}_{3}}>RH\]
(ii) An electron releasing substituent (+ I effect) stabilizes negative charge on the anion resulting in the decrease of stability and thus decreased the acidity of acid.
Electron with drawing nature of halogen : F > Cl > Br > I
Thus, the acidic strength decreases in the order :
\[FC{{H}_{2}}COOH>ClC{{H}_{2}}COOH>BrC{{H}_{2}}COOH>IC{{H}_{2}}COOH\]
similarly :
\[CC{{l}_{3}}COOH>CHC{{l}_{2}}COOH>C{{H}_{2}}ClCOOH>C{{H}_{3}}COOH\]
(iii) Inductive effect is stronger at \[\alpha -\]position than \[\beta -\]position similarly at \[\beta -\]position it is more stronger than at \[\gamma -\]position
Example:
\[C{{H}_{3}}-C{{H}_{2}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-COOH>C{{H}_{3}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-COOH\] \[>\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-C{{H}_{2}}-C{{H}_{2}}-COOH\]
(iv) Relative acid strength in different compounds
\[RCOOH>HOH>ROH>HC\equiv CH>N{{H}_{3}}>RH\]
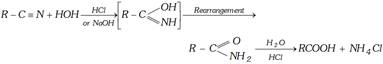 (ii) Hydrolysis of Esters
\[\underset{\text{Ester}}{\mathop{RCOOR'}}\,+HOH\underset{O{{H}^{-}}}{\mathop{\xrightarrow{HCl}}}\,\underset{\text{Acid}}{\mathop{RCOOH}}\,+\underset{\text{Alcohol}}{\mathop{R'OH}}\,\]
(iii) Hydrolysis of Anhydrides
\[\underset{\text{Ethanoic anhydride}}{\mathop{\begin{matrix} C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\, \\ C{{H}_{3}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\, \\ \end{matrix}\ \ \ \ \ O+HOH}}\,\xrightarrow{{{H}^{+}}/O{{H}^{-}}}\underset{\text{Ethanoic acid}}{\mathop{2C{{H}_{3}}COOH}}\,\]
(iv) Hydrolysis of acid chloride and nitro alkane
\[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-Cl+HOH\xrightarrow{{{H}^{+}}/O{{H}^{-}}}RCOOH+HCl\]
\[R-C{{H}_{2}}-N{{O}_{2}}\xrightarrow{85%{{H}_{2}}S{{O}_{4}}}RCOOH\]
(v) Hydrolysis of Trihalogen :
(ii) Hydrolysis of Esters
\[\underset{\text{Ester}}{\mathop{RCOOR'}}\,+HOH\underset{O{{H}^{-}}}{\mathop{\xrightarrow{HCl}}}\,\underset{\text{Acid}}{\mathop{RCOOH}}\,+\underset{\text{Alcohol}}{\mathop{R'OH}}\,\]
(iii) Hydrolysis of Anhydrides
\[\underset{\text{Ethanoic anhydride}}{\mathop{\begin{matrix} C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\, \\ C{{H}_{3}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\, \\ \end{matrix}\ \ \ \ \ O+HOH}}\,\xrightarrow{{{H}^{+}}/O{{H}^{-}}}\underset{\text{Ethanoic acid}}{\mathop{2C{{H}_{3}}COOH}}\,\]
(iv) Hydrolysis of acid chloride and nitro alkane
\[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-Cl+HOH\xrightarrow{{{H}^{+}}/O{{H}^{-}}}RCOOH+HCl\]
\[R-C{{H}_{2}}-N{{O}_{2}}\xrightarrow{85%{{H}_{2}}S{{O}_{4}}}RCOOH\]
(v) Hydrolysis of Trihalogen :
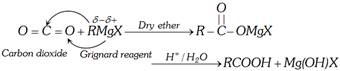
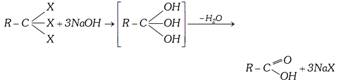 (3) From Grignard Reagent
(3) From Grignard Reagent
 (4) From Alkene or Hydro-carboxy-addition (koch reaction)
\[C{{H}_{2}}=C{{H}_{2}}+CO+{{H}_{2}}O\underset{\begin{smallmatrix}500-1000atm \\ \And 350{}^\circ C \end{smallmatrix}}{\mathop{\xrightarrow{{{H}_{3}}P{{O}_{4}}}}}\,C{{H}_{3}}C{{H}_{2}}COOH\]
(5) Special methods
(i) Carboxylation of sodium alkoxide
\[\underset{\text{Sod}\text{. alkoxide}}{\mathop{RONa+CO}}\,\to \underset{\text{Sod}\text{. salt}}{\mathop{RCOONa}}\,\xrightarrow{HCl}\underset{\text{Acid}}{\mathop{RCOOH}}\,\]
(ii) Action of heat on dicarboxylic acid
(4) From Alkene or Hydro-carboxy-addition (koch reaction)
\[C{{H}_{2}}=C{{H}_{2}}+CO+{{H}_{2}}O\underset{\begin{smallmatrix}500-1000atm \\ \And 350{}^\circ C \end{smallmatrix}}{\mathop{\xrightarrow{{{H}_{3}}P{{O}_{4}}}}}\,C{{H}_{3}}C{{H}_{2}}COOH\]
(5) Special methods
(i) Carboxylation of sodium alkoxide
\[\underset{\text{Sod}\text{. alkoxide}}{\mathop{RONa+CO}}\,\to \underset{\text{Sod}\text{. salt}}{\mathop{RCOONa}}\,\xrightarrow{HCl}\underset{\text{Acid}}{\mathop{RCOOH}}\,\]
(ii) Action of heat on dicarboxylic acid
You need to login to perform this action.
You will be redirected in
3 sec
