| Parent saturated hydrocarbon | Name of the alkyl group | Structure |
| Methane | Methyl | \[C{{H}_{3}}-\] |
| Ethane | Ethyl | \[C{{H}_{3}}-C{{H}_{2}}-\] |
| Propane | n-Propyl | \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\] |
| Butane | n-Butyl | \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\] |
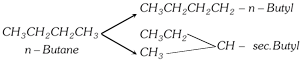 Similarly, removal of different H atoms in pentane gives the following radicals :
\[\underset{n-Pentyl}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}-}}\,\];\[\underset{\text{Isopentyl}}{\mathop{\underset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CHC{{H}_{2}}C{{H}_{2}}-}}\,}}\,}}\,\]; \[\underset{\text{Neopentyl}}{\mathop{\underset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{|\,\,\,}{\mathop{\overset{\,\,\,\,C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,}{\mathop{C{{H}_{3}}CC{{H}_{2}}-}}\,}}\,}}\,}}\,}}\,\];\[\underset{\sec -\text{Pentyl}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CHC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,}}\,\]; \[\underset{tert-\text{Pentyl}}{\mathop{\overset{\text{C}{{\text{H}}_{\text{3}}}\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CC{{H}_{2}}C{{H}_{3}}}}\,}}\,}}\,}}\,\] Unsaturated groups or radicals
Similarly, removal of different H atoms in pentane gives the following radicals :
\[\underset{n-Pentyl}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}-}}\,\];\[\underset{\text{Isopentyl}}{\mathop{\underset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CHC{{H}_{2}}C{{H}_{2}}-}}\,}}\,}}\,\]; \[\underset{\text{Neopentyl}}{\mathop{\underset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{|\,\,\,}{\mathop{\overset{\,\,\,\,C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,}{\mathop{C{{H}_{3}}CC{{H}_{2}}-}}\,}}\,}}\,}}\,}}\,\];\[\underset{\sec -\text{Pentyl}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CHC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,}}\,\]; \[\underset{tert-\text{Pentyl}}{\mathop{\overset{\text{C}{{\text{H}}_{\text{3}}}\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CC{{H}_{2}}C{{H}_{3}}}}\,}}\,}}\,}}\,\] Unsaturated groups or radicals
| Compound | Common name | Compound | Common name | ||||||
| \[C{{H}_{4}}\] | Methane | \[CHC{{l}_{3}}\] | Chloroform | ||||||
| \[{{C}_{2}}{{H}_{2}}\] | Acetylene | \[CH{{I}_{3}}\] | Iodoform | ||||||
| \[{{H}_{3}}CC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\] | n-Butane | \[C{{H}_{3}}CN\] | Acetonitrile | ||||||
| \[{{({{H}_{3}}C)}_{2}}CHC{{H}_{3}}\] | Isobutane | \[C{{H}_{3}}COOH\] | Acetic acid | ||||||
| \[{{({{H}_{3}}C)}_{4}}C\] | Neopentane | \[{{C}_{6}}{{H}_{6}}\] | Benzene | ||||||
| \[HCHO\] | Formaldehyde | \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\] |
Toluene more...
(1) Carbon fibres : These fibres are stronger than steel, stiffer than titanium and lighter than aluminium. Carbon fibres are produced in a number of ways, and from a variety of starting materials or precursors such as viscose rayon, polyacrylonitrile, pitch, resins, gases such as (methane, and benzene). Their characteristics are strongly influenced by the manufacturing techniques employed.
Carbon fibres reinforced in a light weight matrix, generally an epoxy resin, polyester resin or polyamide, are called Carbon Fibre Reinforced Plastics (CFRP). When the carbon fibres are reinforced in a carbon matrix, they are known as Carbon Fibre Reinforced Carbon (CFRC), commonly known as carbon-carbon composites.
On the basis of the characteristics of carbon fibres, carbon firbre reinforced plastics (CFRP) and carbon fibre reinforced carbons (CFRC), their applications can be broadly classified into three categories,
(i) High technology sector including aerospace, military and nuclear fields.
(ii) General engineering sector including sports, transportation and chemical fields.
(iii) Biomedical sector.
Carbon fibres in India are mainly used in defence sector as nose tips and head shields of missiles (like 'Agni') by DRDO, Hyderabad, and in the aerospace sector by ISRO and other aerospace organizations for producing components parts, nozzles of rockets/missiles.
(2) Ceramics : The term ceramics comes from the Greek word keramikos which means burnt stuff, indicating thereby, that desirable properties of these materials are normally achieved through a high-temperature heat treatment process called firing. In the past, the most important materials in this class were the traditional ceramics, prepared from clay, (kaoloinite) a silicate. In the category of traditional ceramics we have porcelain, bricks, tiles, glass and temperature resistant ceramics.
Most ceramic materials fall into an application-classification scheme which is given below,
(i) Clay products : Porcelain, pottery, tablewares, sanitary fittings, building bricks, tiles and sewer pipes.
(ii) Glass ceramics : Kitchenware.
(iii) Refractory materials : Refractory bricks used as furnace linings.
(iv) Abrasive ceramics : Cutting and grinding tools. (familiar examples are silicon and tungsten carbides).
Recently, a family of ceramics have been found to be superconductors with high critical temperatures. One such material is yttrium, barium, copper oxide, which has a critical temperature of about 92 K. New super conduction ceramic materials reported to have even higher critical temperatures have been and are currently being developed. Several of these materials and their critical temperatures are listed below,
Super conducting ceramic materials and their critical temperatures.
|