| K | Ca | Sc | Ti | V | Cr | Mn |
| 227 | 197 | 144 | 132 | 122 | 117 | 117 |
| Fe | Co | Ni | Cu | Zn | Ga | Ge |
| 117 | 116 | 115 | 117 | 125 | 135 | 122* |
| Element | Symbol | At. No. | Electronic configuration | ||||||||||
| Scandium | Sc | 21 |
3d-orbitals are filled up
|
\[\left[ Ar \right]\text{ }3{{d}^{1}}4{{s}^{2}}\] | |||||||||
| Titanium | Ti | 22 | \[\left[ Ar \right]\text{ }3{{d}^{2}}4{{s}^{2}}\] | ||||||||||
| Vanadium | more...
Oxygen is the first member of group 16 or VIA of the periodic table. It consists of five elements Oxygen (O), sulphur (S), selenium (Se), tellurium (Te) and polonium (Po). These (except polonium) are the ore forming elements and thus called chalcogens.
(1) Electronic configuration
Hydrogen peroxide \[({{H}_{2}}{{O}_{2}})\] was discovered by French chemist Thenard.
(1) Preparation : It is prepared by
(i) Laboratory method : In laboratory, \[{{H}_{2}}{{O}_{2}}\] is prepared by Merck?s process. It is prepared by adding calculated amounts of sodium peroxide to ice cold dilute (20%) solution of \[{{H}_{2}}S{{O}_{4}}\].
\[N{{a}_{2}}{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
(ii) By the action of sulphuric acid or phosphoric acid on hydrated barium peroxide \[Ba{{O}_{2}}.8{{H}_{2}}O\]
(a) \[Ba{{O}_{2}}.8{{H}_{2}}O+{{H}_{2}}S{{O}_{4}}\to BaS{{O}_{4}}\downarrow +{{H}_{2}}{{O}_{2}}+8{{H}_{2}}O\]
It must be noted that anhydrous barium peroxide does not react readily with sulphuric acid (because a coating of insoluble barium sulphate is formed on its surface which stops further action of the acid). Therefore, hydrated barium peroxide, \[Ba{{O}_{2}}.8{{H}_{2}}O\] must be used.
(b) \[3Ba{{O}_{2}}+2{{H}_{3}}P{{O}_{4}}\to B{{a}_{3}}{{(P{{O}_{4}})}_{2}}+3{{H}_{2}}{{O}_{2}}\]
\[B{{a}_{3}}{{(P{{O}_{4}})}_{2}}+3{{H}_{2}}S{{O}_{4}}\to 3BaS{{O}_{4}}+2{{H}_{3}}P{{O}_{4}}\]
Phosphoric acid is preferred to \[{{H}_{2}}S{{O}_{4}}\] because soluble impurities like barium persulphate (from \[Ba{{O}_{2}}.8{{H}_{2}}O+{{H}_{2}}S{{O}_{4}}\]) tends to decompose \[{{H}_{2}}{{O}_{2}}\] while \[{{H}_{3}}P{{O}_{4}}\] acts as preservative (negative catalyst) for \[{{H}_{2}}{{O}_{2}}\].
(iii) Industrial method : On a commercial scale, \[{{H}_{2}}{{O}_{2}}\] can be prepared by the electrolysis of 50% \[{{H}_{2}}S{{O}_{4}}\] solution. In a cell, peroxy disulphuric acid is formed at the anode.
\[2{{H}_{2}}S{{O}_{4}}\xrightarrow[\text{Elecrolysis}]{}\underset{\begin{smallmatrix} \text{Peroxy disulphuric} \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{acid} \end{smallmatrix}}{\mathop{{{H}_{2}}{{S}_{2}}{{O}_{8}}(aq.)}}\,+{{H}_{2}}(g)\]
This is drawn off from the cell and hydrolysed with water to give \[{{H}_{2}}{{O}_{2}}\].
\[{{H}_{2}}{{S}_{2}}{{O}_{8}}+2{{H}_{2}}O\xrightarrow{{}}2{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
The resulting solution is distilled under reduced pressure when \[{{H}_{2}}{{O}_{2}}\] gets distilled while \[{{H}_{2}}S{{O}_{4}}\] with high boiling point, remains undistilled.
(iv) By redox process : Industrially \[{{H}_{2}}{{O}_{2}}\] is prepared by the auto-oxidation of 2-alkylanthraquinols. The process involves a cycle of reactions. The net reaction is the catalytic union of \[{{H}_{2}}\] and \[{{O}_{2}}\] to give \[{{H}_{2}}{{O}_{2}}\].
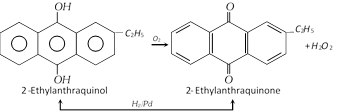 The \[{{H}_{2}}{{O}_{2}}\] formed (about 1%) is extracted with water and concentrated.
(2) Physical properties
(i) Pure hydrogen peroxide is a pale blue syrupy liquid.
(ii) It freezes at - 0.5°C and has a density of 1.4 in pure state.
(iii) Hydrogen peroxide is diamagnetic.
(iv) It is more highly associated via hydrogen bonding than water.
(v) Although it is a better polar solvent than \[{{H}_{2}}O\].
However, it can't be used as such because of strong autooxidation ability.
(vi) Dipole moment of \[{{H}_{2}}{{O}_{2}}\] is 2.1 D.
(3) Chemical properties
(i) Decomposition : Pure \[{{H}_{2}}{{O}_{2}}\] is an unstable liquid and decomposes into water and \[{{O}_{2}}\] either upon standing or upon heating, \[2{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}};\,\,\,\Delta H=-196.0\,kJ\]
(ii) Oxidising nature : It is a powerful oxidising agent. It acts as an oxidising agent in neutral, acidic or in alkaline medium. e.g. \[2KI+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2KOH+{{I}_{2}}\] [In neutral medium]
\[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+2{{H}_{2}}O\] [In acidic medium]
\[MnS{{O}_{4}}+{{H}_{2}}{{O}_{2}}+2NaOH\xrightarrow{{}}Mn{{O}_{2}}+N{{a}_{2}}S{{O}_{4}}+2{{H}_{2}}O\]
[In alkaline medium]
(iii) Reducing nature : \[{{H}_{2}}{{O}_{2}}\] has tendency to take up oxygen from strong oxidising agents and thus, acts as a reducing agent,
\[\underset{\begin{smallmatrix} \text{From oxidising } \\ \,\,\,\,\,\,\,\,\,\,\text{agent} \end{smallmatrix}}{\mathop{{{H}_{2}}{{O}_{2}}+O\xrightarrow{{}}{{H}_{2}}O+{{O}_{2}}}}\,\]. It can act as a reducing agent in acidic, basic or even neutral medium.
In acidic medium, \[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}^{+}}+{{O}_{2}}+2{{e}^{-}}\]
In alkaline medium,
\[{{H}_{2}}{{O}_{2}}+2O{{H}^{-}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}}+2{{e}^{-}}\]
(iv) Bleaching action : \[{{H}_{2}}{{O}_{2}}\] acts as a bleaching agent due to the release of nascent oxygen.
\[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O+O\]
Thus, the bleaching action of \[{{H}_{2}}{{O}_{2}}\] is due to oxidation. It oxidises the colouring matter to a colourless product, Colouring matter + O \[\to \] Colour less matter.
\[{{H}_{2}}{{O}_{2}}\] is more...
The \[{{H}_{2}}{{O}_{2}}\] formed (about 1%) is extracted with water and concentrated.
(2) Physical properties
(i) Pure hydrogen peroxide is a pale blue syrupy liquid.
(ii) It freezes at - 0.5°C and has a density of 1.4 in pure state.
(iii) Hydrogen peroxide is diamagnetic.
(iv) It is more highly associated via hydrogen bonding than water.
(v) Although it is a better polar solvent than \[{{H}_{2}}O\].
However, it can't be used as such because of strong autooxidation ability.
(vi) Dipole moment of \[{{H}_{2}}{{O}_{2}}\] is 2.1 D.
(3) Chemical properties
(i) Decomposition : Pure \[{{H}_{2}}{{O}_{2}}\] is an unstable liquid and decomposes into water and \[{{O}_{2}}\] either upon standing or upon heating, \[2{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}};\,\,\,\Delta H=-196.0\,kJ\]
(ii) Oxidising nature : It is a powerful oxidising agent. It acts as an oxidising agent in neutral, acidic or in alkaline medium. e.g. \[2KI+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2KOH+{{I}_{2}}\] [In neutral medium]
\[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+2{{H}_{2}}O\] [In acidic medium]
\[MnS{{O}_{4}}+{{H}_{2}}{{O}_{2}}+2NaOH\xrightarrow{{}}Mn{{O}_{2}}+N{{a}_{2}}S{{O}_{4}}+2{{H}_{2}}O\]
[In alkaline medium]
(iii) Reducing nature : \[{{H}_{2}}{{O}_{2}}\] has tendency to take up oxygen from strong oxidising agents and thus, acts as a reducing agent,
\[\underset{\begin{smallmatrix} \text{From oxidising } \\ \,\,\,\,\,\,\,\,\,\,\text{agent} \end{smallmatrix}}{\mathop{{{H}_{2}}{{O}_{2}}+O\xrightarrow{{}}{{H}_{2}}O+{{O}_{2}}}}\,\]. It can act as a reducing agent in acidic, basic or even neutral medium.
In acidic medium, \[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}^{+}}+{{O}_{2}}+2{{e}^{-}}\]
In alkaline medium,
\[{{H}_{2}}{{O}_{2}}+2O{{H}^{-}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}}+2{{e}^{-}}\]
(iv) Bleaching action : \[{{H}_{2}}{{O}_{2}}\] acts as a bleaching agent due to the release of nascent oxygen.
\[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O+O\]
Thus, the bleaching action of \[{{H}_{2}}{{O}_{2}}\] is due to oxidation. It oxidises the colouring matter to a colourless product, Colouring matter + O \[\to \] Colour less matter.
\[{{H}_{2}}{{O}_{2}}\] is more... Current Affairs CategoriesArchive
Trending Current Affairs
You need to login to perform this action. | ||||||||||||