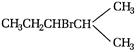question_answer 1) According to Huygen's principle, the locus of all the particles of the medium, which at any instant are vibrating in the same phase, is called
A)
half period zone
done
clear
B)
oscillator
done
clear
C)
wave front
done
clear
D)
ray of light
done
clear
View Answer play_arrow
question_answer 2) In air the value of the total electric flux emitted from unit positive charge is
A)
\[{{\varepsilon }_{0}}\]
done
clear
B)
\[{{({{\varepsilon }_{0}})}^{-1}}\]
done
clear
C)
\[{{(4\pi {{\varepsilon }_{0}})}^{-1}}\]
done
clear
D)
\[4\pi {{\varepsilon }_{0}}\]
done
clear
View Answer play_arrow
question_answer 3) A rod AB is 1 m long. The temperature of its one end A is maintained at \[100{}^\circ C\] and other end B at WC. the temperature at a distance of 60 cm from point B is
A)
\[64{}^\circ C\]
done
clear
B)
\[36{}^\circ C\]
done
clear
C)
\[46{}^\circ C\]
done
clear
D)
\[72{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 4) An oil on the water surface spreads like a thin layer because
A)
oil drop tries to become spherical due to the surface tension
done
clear
B)
surface tension of water is greater than the surface tension of oil
done
clear
C)
surface tension of water is equal to the surface of oil
done
clear
D)
weight of oil is less than weight of water
done
clear
View Answer play_arrow
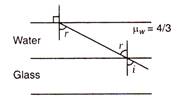
question_answer 5)
A ray of light is incident on the interface between water and glass at an angle i and retracted parallel to the water surface, then value of \[{{\mu }_{g}}\]will be
A)
\[(4/3)\sin i\]
done
clear
B)
\[\frac{1}{\sin i}\]
done
clear
C)
\[4/3\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 6) Find the ratio of electric field to potential EIV at the midpoint of electric dipole, if the distance between the charges is \[l\].
A)
\[1/l\]
done
clear
B)
\[l\]
done
clear
C)
\[2/l\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 7) Which diode is forward bias?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
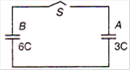
question_answer 8)
In the given circuit when key S is closed then charge on the capacitors A and B are respectively
A)
3q, 6q
done
clear
B)
6q, 3q
done
clear
C)
4.5q, 4.5q
done
clear
D)
5q, 4q
done
clear
View Answer play_arrow
question_answer 9)
The intensity distribution of X-rays from two coolidge tubes operated on different voltages
A)
\[{{V}_{1}}>{{V}_{2}}\]and \[{{Z}_{1}}<{{Z}_{2}}\]
done
clear
B)
\[{{V}_{1}}>{{V}_{2}}\]and \[{{Z}_{1}}>{{Z}_{2}}\]
done
clear
C)
\[{{V}_{1}}<{{V}_{2}}\]and\[{{Z}_{1}}>{{Z}_{2}}\]
done
clear
D)
\[{{V}_{1}}={{V}_{2}}\]and\[{{Z}_{1}}<{{Z}_{2}}\]
done
clear
View Answer play_arrow
question_answer 10) The binding energy of deuteron\[_{1}^{2}H\]is 1.112 MeV per nucleon and an \[\alpha \]-particle\[_{2}^{4}He\]has a binding energy of 7.047 MeV per nucleon. Then is the fusion reaction\[_{1}^{2}H+_{1}^{2}H\xrightarrow[{}]{{}}_{2}^{4}H+Q,\]the energy Q released is
A)
1 MeV
done
clear
B)
11.9 MeV
done
clear
C)
23.8 MeV
done
clear
D)
931 MeV
done
clear
View Answer play_arrow
question_answer 11) How many second will light take to cover a distance of one fermi?
A)
\[{{10}^{-15}}s\]
done
clear
B)
\[3\times {{10}^{8}}s\]
done
clear
C)
\[3.33\times {{10}^{-24}}s\]
done
clear
D)
\[3.3\times {{10}^{7}}s\]
done
clear
View Answer play_arrow
question_answer 12) A body is moved in straight line by constant power of machine. What will be the relation the travelling distance and time?
A)
\[{{s}^{2}}\alpha \,{{t}^{3}}\]
done
clear
B)
\[{{s}^{2}}\alpha \,{{t}^{3}}\]
done
clear
C)
\[{{s}^{3}}\alpha \,{{t}^{2}}\]
done
clear
D)
\[s\,\alpha \,{{t}^{3}}\]
done
clear
View Answer play_arrow
question_answer 13) The square of resultant of two equal forces is three times of their product. The angle between them \[\overrightarrow{P}\] and \[\overrightarrow{Q}\] will be
A)
\[\pi /3\]
done
clear
B)
\[\pi /4\]
done
clear
C)
\[\pi /5\]
done
clear
D)
\[\pi /6\]
done
clear
View Answer play_arrow
question_answer 14) A body placed on the earth at static position. If it is pushed then the final state of centre of gravity will be
A)
near the earth
done
clear
B)
at some height from the earth
done
clear
C)
remain same
done
clear
D)
remain at the same plane
done
clear
View Answer play_arrow
question_answer 15) The particle of mass 50 kg is at rest. The work done to accelerates it by 20 in/s in 10 s is
A)
\[{{10}^{3}}J\]
done
clear
B)
\[{{10}^{4}}J\]
done
clear
C)
\[2\times {{10}^{3}}J\]
done
clear
D)
\[4\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 16) The moment of inertia of a circular loop of radius R, at a distance of R/2 around a rotating axis parallel to horizontal diameter of loop is
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
C)
\[2M{{R}^{2}}\]
done
clear
D)
\[\frac{3}{4}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 17) If the earth stops moving around its polar axis then what will be effect on body placed at south axis?
A)
Remain same
done
clear
B)
Increase
done
clear
C)
Decrease but not zero
done
clear
D)
Decrease zero
done
clear
View Answer play_arrow
question_answer 18) In designing a beam for its use to support a load. The depression at centre is proportional to (where Y is Young's modulus)
A)
\[{{Y}^{2}}\]
done
clear
B)
Y
done
clear
C)
\[\frac{1}{Y}\]
done
clear
D)
\[\frac{1}{{{Y}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 19) The temperature of sun is
A)
1000 K
done
clear
B)
7000 K
done
clear
C)
10 K
done
clear
D)
\[10\times {{10}^{6}}K\]
done
clear
View Answer play_arrow
question_answer 20) A balloon is filled at\[27{}^\circ C\]and 1 atm pressure by 500 m3 He. At\[~-3{}^\circ C\]and 0.5 atm pressure, the volume of He will be
A)
\[700\text{ }{{m}^{3}}\]
done
clear
B)
\[900\text{ }{{m}^{3}}\]
done
clear
C)
\[1000\text{ }{{m}^{3}}\]
done
clear
D)
\[500\text{ }{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 21) Which among the following is different from other's?
A)
Wavelength
done
clear
B)
Velocity
done
clear
C)
Frequency
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 22) The time period of two pendulums are T and 5T/4. They starts motion in SHM from equilibrium position. What will be the phase difference between them, after one complete oscillation by the big pendulum?
A)
\[45{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 23) Hydrogen gas is filled in the bubble. For the sound wave, the bubble will be
A)
like a converging lens
done
clear
B)
like a convex mirror
done
clear
C)
like a concave lens
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 24) Two charges are at a distance r exert a force F on each other. If the charges are doubled and distance between them is halved, then the new force acting on each charge is
A)
\[\frac{F}{8}\]
done
clear
B)
\[\frac{F}{4}\]
done
clear
C)
\[4F\]
done
clear
D)
\[16\,F\]
done
clear
View Answer play_arrow
question_answer 25) The ratio of radius of two bubbles is 2 : 1. What is the ratio excess pressure inside them
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
2 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 26) Brownian motion is evidence of
A)
kinetic energy of substance
done
clear
B)
principle of electromagnetic
done
clear
C)
Newton's law
done
clear
D)
photoelectric phenomenon
done
clear
View Answer play_arrow
question_answer 27) Beats are result of
A)
interference
done
clear
B)
refraction
done
clear
C)
reflection
done
clear
D)
diffraction
done
clear
View Answer play_arrow
question_answer 28) An ice block contains a glass ball when the see melts within the water containing vessel, the level of water
A)
will increase
done
clear
B)
will decrease
done
clear
C)
will remain same
done
clear
D)
first increase and after that decrease
done
clear
View Answer play_arrow
question_answer 29) Two bodies of masses 2 kg and 4 kg having velocities 2 m/s and 10 m/s respectively are coming close to each other under a gravitational force, then the velocity of centre of mass will be
A)
5 m/s
done
clear
B)
6 m/s
done
clear
C)
8 m/s
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 30) The displacement of oscillating body is given by\[y=A\text{ }sin(B+Ct+D)\]. The dimensions of ABCD are
A)
\[\left[ {{M}^{0}}{{L}^{-1}}T \right]\]
done
clear
B)
\[\left[ {{M}^{0}}{{L}^{0}}{{T}^{-1}} \right]\]
done
clear
C)
\[\left[ {{M}^{0}}{{L}^{^{-1}}}{{T}^{^{-1}}} \right]\]
done
clear
D)
\[\left[ {{M}^{0}}{{L}^{0}}{{T}^{0}} \right]\]
done
clear
View Answer play_arrow
question_answer 31) The ratio of intensities of two beams of light in interference is 9 : 1. The ratio of maximum and minimum intensities
A)
10 : 8
done
clear
B)
9 : 1
done
clear
C)
4 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 32) Four wires having same length, diameter and material are connected in the form of a square. If the resistance of each wire is R, then resistance of combination across any two opposite ends is
A)
R
done
clear
B)
R/2
done
clear
C)
R/4
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) The electric motor operating on a 200 volt DC supply draw a current of 10 A. If the power is 40%, then resistance of motor
A)
20
done
clear
B)
8 n
done
clear
C)
12 n
done
clear
D)
16 n
done
clear
View Answer play_arrow
question_answer 34) A capacitor of capacitance 2.0 \[\mu \]F is charged upto 200 V and then the plates of capacitor are connected to a resistance wire. The heat released in joule will be
A)
\[2\times {{10}^{-2}}\]
done
clear
B)
\[4\times {{10}^{-2}}\]
done
clear
C)
\[4\times {{10}^{4}}\]
done
clear
D)
\[2\times {{10}^{10}}\]
done
clear
View Answer play_arrow
question_answer 35) A voltmeter having a resistance of\[998\,\Omega \]is connected to a cell of emf 2 V and internal resistance\[2\,\Omega \]. The error in the measurement of emf will be
A)
\[4\times {{10}^{-1}}V\]
done
clear
B)
\[2\times {{10}^{-3}}V\]
done
clear
C)
\[4\times {{10}^{-3}}V\]
done
clear
D)
\[2\times {{10}^{-1}}V\]
done
clear
View Answer play_arrow
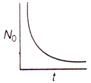
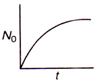
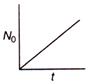
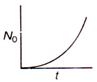
question_answer 36) The graph between the instantaneous concentration (N) of a radioactive element and time (t) is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 37) What is increased in step down transformer?
A)
current
done
clear
B)
voltage
done
clear
C)
power
done
clear
D)
frequency
done
clear
View Answer play_arrow
question_answer 38) According to modern physics the nature of light is
A)
only wave
done
clear
B)
only particle
done
clear
C)
Both wave and particle
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 39) Which has more luminous efficiency?
A)
a 40 W bulb
done
clear
B)
a 40 W tube light
done
clear
C)
Both of same
done
clear
D)
Cannot say
done
clear
View Answer play_arrow
question_answer 40) When a light ray is travelling from one medium to another velocity of light ray doubles. If no total internal reflection occurs then the maximum value of incident angle will be
A)
\[60{}^\circ \]
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 41) Photon and electron are given same energy\[({{10}^{-20}}J)\]. Wavelength associated with photon and electron are\[{{\lambda }_{p}}\]and\[{{\lambda }_{e}}\]the correct statement will be
A)
\[{{\lambda }_{p}}>{{\lambda }_{e}}\]
done
clear
B)
\[{{\lambda }_{p}}<{{\lambda }_{e}}\]
done
clear
C)
\[{{\lambda }_{p}}={{\lambda }_{e}}\]
done
clear
D)
\[\frac{{{\lambda }_{e}}}{{{\lambda }_{p}}}=c\]
done
clear
View Answer play_arrow
question_answer 42) The half-life of radioactive element is 600 yr. The fraction of sample that would remain after 3000 yr is
A)
1 / 2
done
clear
B)
1 / 16
done
clear
C)
1 / 8
done
clear
D)
1 / 32
done
clear
View Answer play_arrow
question_answer 43) The light of wavelength 5000\[\overset{o}{\mathop{\text{A}}}\,\]falls on a photosensitive plate of work function 1.9 eV. The kinetic energy of the emitted photoelectron is
A)
0.58 eV
done
clear
B)
2.48 eV
done
clear
C)
1.24 eV
done
clear
D)
1.18 eV
done
clear
View Answer play_arrow
question_answer 44) The number of electrons and holes in semiconductor at normal temperature will be
A)
equal
done
clear
B)
zero
done
clear
C)
unequal
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 45) The unit of thermal conductivity is
A)
\[W{{m}^{-1}}{{K}^{-1}}\]
done
clear
B)
\[J{{K}^{-1}}\]
done
clear
C)
\[WmK\]
done
clear
D)
\[JK\]
done
clear
View Answer play_arrow
question_answer 46) If vector \[2\widehat{i}-\widehat{j}+\widehat{k},\widehat{i}+2\widehat{j}-3\widehat{k}\]and \[3\widehat{i}+p\widehat{j}+5\widehat{k}\] are coplanar, find the value of p
A)
16
done
clear
B)
\[-4\]
done
clear
C)
4
done
clear
D)
\[-8\]
done
clear
View Answer play_arrow
question_answer 47) In the capacitor of capacitance C, charge Q and energy W is stored. If charge is increased upto 2Q, the energy stored will be
A)
\[\frac{W}{4}\]
done
clear
B)
\[\frac{W}{2}\]
done
clear
C)
2 W
done
clear
D)
4 W
done
clear
View Answer play_arrow
question_answer 48) At 300 K, the concentration of electron (He) and hole\[({{n}_{h}})\]in pure silicon is\[1.5\times {{10}^{16}}{{m}^{3}}\]. When indium is added,\[{{n}_{h}}\]become\[4.5\times {{10}^{22}}\]. Then\[{{n}_{e}}\]in doped silicon is
A)
\[9\times {{0}^{5}}\]
done
clear
B)
\[5\times {{10}^{9}}\]
done
clear
C)
\[2.25\times {{10}^{11}}\]
done
clear
D)
\[3\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 49) A cylindrical conductors is placed near an another positive conductor. The total charge induced by the conductor is
A)
only positive
done
clear
B)
only negative
done
clear
C)
zero
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 50) If units of force, length and time are 1 kilo-newton, 1 km and 100 second respectively, then unit of mass will be
A)
1000 kg
done
clear
B)
1kg
done
clear
C)
10000 kg
done
clear
D)
100 kg
done
clear
View Answer play_arrow
question_answer 51) Which of the following compound does not contain carboxylic group\[(-COOH)\]?
A)
Acetic acid
done
clear
B)
Lactic acid
done
clear
C)
Benzoic acid
done
clear
D)
Picric acid
done
clear
View Answer play_arrow
question_answer 52) Which of the following compound will not obtain, on the distillation of calcium acetate with calcium formate?
A)
Acetone
done
clear
B)
Formaldehyde
done
clear
C)
Acetaldehyde
done
clear
D)
Propionaldehyde
done
clear
View Answer play_arrow
question_answer 53) Glucose gives silver mirror test because it contains
A)
\[-COOH\]group
done
clear
B)
a basic group
done
clear
C)
a ketonic group
done
clear
D)
an aldehyde group
done
clear
View Answer play_arrow
question_answer 54) Which compound is used to obtain Teflon polymer?
A)
Difluoro ethane
done
clear
B)
Monofluoro ethane
done
clear
C)
Tetrafluoro ethane
done
clear
D)
Tetrafluoro ethane
done
clear
View Answer play_arrow
question_answer 55) Which of the following equation shows the de-Broglie relationship?
A)
\[\frac{h}{mv}=p\]
done
clear
B)
\[\lambda m=\frac{v}{p}\]
done
clear
C)
\[\lambda =\frac{h}{mp}\]
done
clear
D)
\[\lambda =\frac{h}{mv}\]
done
clear
View Answer play_arrow
question_answer 56) Which of the following will the order of energy of subshells related to principal quantum number\[(n=4)\]?
A)
\[s<p<d<f\]
done
clear
B)
\[s<d<p<f\]
done
clear
C)
\[s<f<p<d\]
done
clear
D)
\[p<s<d<f\]
done
clear
View Answer play_arrow
question_answer 57) The electron shell is not spherical in, which of the following element?
A)
He
done
clear
B)
Be
done
clear
C)
B
done
clear
D)
Li
done
clear
View Answer play_arrow
question_answer 58) The correct electronic configuration of iron is
A)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{2}},3{{d}^{6}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{2}},3{{d}^{5}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{2}},3{{d}^{7}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{1}},3{{d}^{5}}\]
done
clear
View Answer play_arrow
question_answer 59) According to VSEPR theory the geometry of water molecule is
A)
octahedral
done
clear
B)
distorted tetrahedral
done
clear
C)
trigonal planar
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 60) On adding a non-volatile solution in a solvent, the vapour pressure of solvent reduces 10 mm of Hg. The mole fraction of solute is 0.2 in the solution. The vapour pressure of solution reduces to 20 mm of Hg on adding more solute in the solution. Now, what will the mole fraction of solvent in solution?
A)
0.2
done
clear
B)
0.4
done
clear
C)
0.6
done
clear
D)
0.8
done
clear
View Answer play_arrow
question_answer 61) Which of the following statement is false for ionic crystals?
A)
The boiling point and melting point of ionic crystals are high.
done
clear
B)
These dissolves in water and other solvents.
done
clear
C)
At low temperature, these are conductor of electricity in solid state.
done
clear
D)
Their cohesive energy is high.
done
clear
View Answer play_arrow
question_answer 62) 8 g amount of a radioactive substance remains 0.5 g after 1 h. What is its half-life?
A)
10 min
done
clear
B)
15 min
done
clear
C)
30 min
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 63) In a radioactive change \[R\xrightarrow[{}]{-a}X\xrightarrow[{}]{-\beta }Y\xrightarrow[{}]{-\beta }Z\] R and Z are
A)
isotope
done
clear
B)
isobar
done
clear
C)
isomer
done
clear
D)
isoneutronic
done
clear
View Answer play_arrow
question_answer 64) The dissociation of\[X{{Y}_{2}}\]is as \[X{{Y}_{2}}(g)XY(g)+Y(g)\] The initial pressure of\[X{{Y}_{2}}\]is 600 mm of\[Hg\]. On establishing the equilibrium the total pressure becomes 800 mm of Hg. What is the value of K for the reaction, when the volume of system remains unchanged?
A)
50
done
clear
B)
100
done
clear
C)
166.6
done
clear
D)
150
done
clear
View Answer play_arrow
question_answer 65) The quantity of ionisation of decinormal solution of\[C{{H}_{3}}COOH\]is 1.3%. What is the pH value of this solution? (log 1.3=0.11)
A)
2.89
done
clear
B)
3.89
done
clear
C)
4.89
done
clear
D)
0.89
done
clear
View Answer play_arrow
question_answer 66) A buffer solution is obtained on adding 10 mL \[1.0\text{ }M\,C{{H}_{3}}COOH\]and 20 mL 0.5\[0.5\text{ }M\,C{{H}_{3}}COONa\]. It is diluted to 100 mL by distilled water. If\[p{{K}_{a}}=4.76\]for\[C{{H}_{3}}COOH,\]then the pH value of buffer solution
A)
2.76
done
clear
B)
3.76
done
clear
C)
4.76
done
clear
D)
0.76
done
clear
View Answer play_arrow
question_answer 67) Hess's law is used in the determination of
A)
heat of reaction
done
clear
B)
heat of formation
done
clear
C)
heat of bond formation
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 68) 1.0 L of 1.0 M sodium hydroxide is neutralized by 1.0 L of 1.0 M methanoic acid. If the heat of formation of water is X then the heat of neutralization of this reaction is
A)
less than X
done
clear
B)
more than X
done
clear
C)
equal to X
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 69) 1.0 L of 2.0 M acetic acid is added with 1.0 L of 3.0 M ethyl alcohol. The following reaction takes place during this process \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\] \[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O\] If each solution is diluted by adding 1.0 L water, then initial rate of the reaction will reduce
A)
0.5 times
done
clear
B)
2.0 times
done
clear
C)
4.0 times
done
clear
D)
0.25 times
done
clear
View Answer play_arrow
question_answer 70) For a reaction, the rate constant is \[0.693\times {{10}^{-1}}mi{{n}^{-1}}\]and initial concentration is 0.2 mol/L. Half-life period will be
A)
400 s
done
clear
B)
600 s
done
clear
C)
800 s
done
clear
D)
100 s
done
clear
View Answer play_arrow
question_answer 71) For chemical reaction \[{{N}_{2}}+3{{H}_{2}}\xrightarrow[{}]{{}}2N{{H}_{3}}\] \[\frac{d[N{{H}_{3}}]}{dt}=2\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\] The value of\[-\frac{d[{{H}_{2}}]}{dt}\]will
A)
\[1\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[3\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[4\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[6\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following unit of energy show the maximum value of energy?
A)
Calorie
done
clear
B)
Joule
done
clear
C)
Erg
done
clear
D)
Electron-volt
done
clear
View Answer play_arrow
question_answer 73) The value of standard reduction potentials of three metallic cations X, Y and Z are + 0.52, \[-3.03\]and\[-1.18\]volt respectively. The order of reducing power of these metal will
A)
\[Y>Z>X\]
done
clear
B)
\[X>Y>Z\]
done
clear
C)
\[X>Z>Y\]
done
clear
D)
\[Z>X>Y\]
done
clear
View Answer play_arrow
question_answer 74) If \[F{{e}^{2+}}+2{{e}^{-1}}\xrightarrow[{}]{{}}Fe;\] \[E{}^\circ =-0.44V\] and \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow[{}]{{}}Zn;\] \[E{}^\circ =-0.76\,V\] which of the following is the correct statement?
A)
Fe is more electropositive
done
clear
B)
Zn is more electropositive
done
clear
C)
Zn is more electronegative
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 75) On the electrolysis of aqueous\[CuS{{O}_{4}}\]solution, by unreactive Pt electrodes, the reaction is occurred at anode
A)
\[2SO_{4}^{2-}\xrightarrow[{}]{{}}{{S}_{2}}O_{3}^{2-}+2\frac{1}{2}{{O}_{2}}+2{{e}^{-}}\]
done
clear
B)
\[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow[{}]{{}}Cu\]
done
clear
C)
\[2{{H}_{2}}O\xrightarrow[{}]{{}}{{O}_{2}}+4{{H}^{+}}+4{{e}^{-}}\]
done
clear
D)
\[2{{H}^{+}}+2{{e}^{-}}\xrightarrow[{}]{{}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following chemical reaction represents homogeneous catalysis?
A)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow[{}]{Fe}2N{{H}_{3}}(g)\]
done
clear
B)
\[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow[{}]{NO}2S{{O}_{3}}(g)\]
done
clear
C)
\[CO(g)+3{{H}_{2}}(g)\xrightarrow[{}]{Ni}C{{H}_{4}}(g)+{{H}_{2}}O(g)\]
done
clear
D)
\[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow[{}]{{{V}_{2}}{{O}_{5}}}2S{{O}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following is correct for the reaction? \[MnO_{4}^{-}+CO_{4}^{2-}+{{H}^{+}}\xrightarrow[{}]{{}}M{{n}^{2+}}+C{{O}_{2}}+{{H}_{2}}O\]
A)
\[M{{n}^{2+}}\] - 5 \[C{{r}_{2}}O_{4}^{2-}\]-2 \[C{{O}_{2}}\]- 4 \[{{H}^{+}}\]-10
done
clear
B)
\[M{{n}^{2+}}\] -2 \[C{{r}_{2}}O_{4}^{2-}\]- 5 \[C{{O}_{2}}\]-10 \[{{H}^{+}}\]-16
done
clear
C)
\[M{{n}^{2+}}\] -6 \[C{{r}_{2}}O_{4}^{2-}\]-8 \[C{{O}_{2}}\]- 16 \[{{H}^{+}}\]-18
done
clear
D)
\[M{{n}^{2+}}\] - 10 \[C{{r}_{2}}O_{4}^{2-}\]- 12 \[C{{O}_{2}}\]-24 \[{{H}^{+}}\]-12
done
clear
View Answer play_arrow
question_answer 78) The oxidation number of Cr is changed on reaction of acidic\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]with\[{{H}_{2}}S\]
A)
+ 3 to + 6
done
clear
B)
+ 6 to + 3
done
clear
C)
+ 6 to + 2
done
clear
D)
remains unchanged
done
clear
View Answer play_arrow
question_answer 79) The oxidation number of oxygen is + 2 in, which of the following compound?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[O{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following is the most electronegative?
A)
Lead
done
clear
B)
Silicon
done
clear
C)
Carbon
done
clear
D)
Tin
done
clear
View Answer play_arrow
question_answer 81) Which of the following is the s-block element?
A)
Aluminium
done
clear
B)
Chromium
done
clear
C)
Carbon
done
clear
D)
Potassium
done
clear
View Answer play_arrow
question_answer 82) Which of the following is the correct order of decreasing ionic radii of ions?
A)
\[{{N}^{3-}}>{{O}^{2-}}>{{F}^{-}}>N{{a}^{+}}\]
done
clear
B)
\[{{N}^{3-}}>N{{a}^{+}}>{{O}^{2-}}>{{F}^{-}}\]
done
clear
C)
\[N{{a}^{+}}>{{O}^{2-}}>{{N}^{3-}}>{{F}^{-}}\]
done
clear
D)
\[N{{a}^{+}}>{{F}^{-}}>{{O}^{2-}}>{{N}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 83) The electronic configuration of a element is as \[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},3{{d}^{10}},4{{s}^{2}},4{{p}^{3}}\]The property of this element is similar to
A)
boron
done
clear
B)
oxygen
done
clear
C)
nitrogen
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 84) Malachite is the ore of
A)
Fe
done
clear
B)
Cu
done
clear
C)
Zn
done
clear
D)
Hg
done
clear
View Answer play_arrow
question_answer 85) For the purification of blister copper, the copper is melted in the furnance and stirring with green logs of woot. The purpose of this
A)
to remove dissolved gases in blister copper
done
clear
B)
by taking the impurities on the surface to oxidise them
done
clear
C)
to increase the quantity of carbon in copper
done
clear
D)
the impurities of metal oxide are reduced by hydrocarbon gases evolved from wood
done
clear
View Answer play_arrow
question_answer 86) In the smelting of a metal ore, a substance is added, which forms a fusible product on mixing with impurities. The name of this substance is
A)
slag
done
clear
B)
mud
done
clear
C)
gangue
done
clear
D)
flux
done
clear
View Answer play_arrow
question_answer 87) Which of the following pair cannot remain together?
A)
\[NaHC{{O}_{3}}\]and\[NaCl\]
done
clear
B)
\[NaHC{{O}_{3}}\]and\[NaOH\]
done
clear
C)
\[NaHC{{O}_{3}}\]and\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\]and\[NaOH\]
done
clear
View Answer play_arrow
question_answer 88) Which of the following has the highest melting point?
A)
Barium
done
clear
B)
Strontium
done
clear
C)
Calcium
done
clear
D)
Radium
done
clear
View Answer play_arrow
question_answer 89) The transition elements are generally
A)
diamagnetic
done
clear
B)
paramagnetic
done
clear
C)
neither diamagnetic nor paramagnetic
done
clear
D)
both diamagnetic and paramagnetic
done
clear
View Answer play_arrow
question_answer 90) The oxidation number of metal is zero in, which of the following co-ordination compound?
A)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
B)
\[[Cr{{(CO)}_{6}}]\]
done
clear
C)
\[[Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
D)
\[[Cr{{(en)}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 91) \[[{{({{C}_{6}}{{H}_{5}})}_{2}}Pd{{(NCS)}_{2}}]\]and\[[{{({{C}_{6}}{{H}_{5}})}_{2}}Pd{{(SCN)}_{2}}]\]are
A)
linkage isomers
done
clear
B)
coordination isomers
done
clear
C)
ionisation isomers
done
clear
D)
geometrical isomers
done
clear
View Answer play_arrow
question_answer 92) The product forms mainly on the fusion of Na with aniline
A)
\[NaCN\]
done
clear
B)
\[Na{{N}_{3}}\]
done
clear
C)
\[NaSCN\]
done
clear
D)
\[NaN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 93) The combustion of liquid benzene in oxygen is as \[2{{C}_{6}}{{H}_{6}}+15{{O}_{2}}\xrightarrow[{}]{{}}12C{{O}_{2}}+6{{H}_{2}}O\] How many litre of oxygen will require on complete combustion of 3.9 g liquid benzene at STP
A)
11.2 L
done
clear
B)
22.4 L
done
clear
C)
8.4 L
done
clear
D)
7.4 L
done
clear
View Answer play_arrow
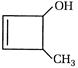
question_answer 94)
The IUPAC name of the following compounds
A)
3-methyl cyclo-l-butene-2-ol
done
clear
B)
4-methyl cyclo-but-2-ene-l-ol
done
clear
C)
4-methyl cyclo-but-l-ene-3-ol
done
clear
D)
2-methyl cyclo-3-butene-l-ol
done
clear
View Answer play_arrow
question_answer 95) Which of the following compound does not show optical isomerism?
A)
\[C{{H}_{3}}CH(OH)Br\]
done
clear
B)
\[C{{H}_{3}}CH(OH)C{{H}_{3}}\]
done
clear
C)
done
clear
D)
\[C{{H}_{3}}-CHOH-CHBr-C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 96) In the following dehydration reaction the hybridised state of carbon changes \[C{{H}_{3}}CON{{H}_{2}}\xrightarrow[{}]{{{P}_{2}}{{O}_{5}}}C{{H}_{3}}CN+{{H}_{2}}O\]
A)
\[s{{p}^{3}}\]to\[s{{p}^{2}}\]
done
clear
B)
\[sp\]to \[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}}\]to\[sp\]
done
clear
D)
\[sp\]to\[s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 97) Acetylene reacts with\[HCN\]in the presence of \[Ba{{(CN)}_{2}}\]to give
A)
vinyl cyanide
done
clear
B)
1,1-dicyano ethane
done
clear
C)
1,2-dicyano ethane
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 98) The substitution of chlorine becomes the most easiest in which of the following compound?
A)
Chloro benzene
done
clear
B)
Vinyl chloride
done
clear
C)
Allyl chloride
done
clear
D)
p-chloro toluene
done
clear
View Answer play_arrow
question_answer 99) Commercially, methanol is manufactured by which of the following method?
A)
Catalytic reduction of CO in the presence of \[ZnO,C{{r}_{2}}{{O}_{3}}\]
done
clear
B)
The reaction of\[C{{H}_{4}}\]with water vapour in the presence of Ni catalyst at\[900{}^\circ C\]
done
clear
C)
By the reaction of formaldehyde with\[LiAl{{H}_{4}}\]
done
clear
D)
By the reaction of\[HCHO\]with aqueous\[KOH\]
done
clear
View Answer play_arrow
question_answer 100) The order of acidic strength of phenol, p-cresol, m-nitrophenol and p-nitrophenol is
A)
phenol, p-cresol, p-nitrophenol, m-nitrophenol
done
clear
B)
p-cresol, phenol, 2n-nitrophenol, p-nitrophenol
done
clear
C)
p-cresol, m-nitrophenol, phenol, p-nitrophenol
done
clear
D)
m-nitrophenol, phenol, p-cresol, p-nitrophenol
done
clear
View Answer play_arrow
question_answer 101) \[\int_{0}^{\pi /2}{\frac{x\sin x.\cos x}{{{\cos }^{4x}}+{{\sin }^{4}}x}dx}\]is equal to
A)
\[\frac{{{\pi }^{2}}}{8}\]
done
clear
B)
\[\frac{{{\pi }^{2}}}{16}\]
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 102) The equation of tangent to the hyperbola \[2{{x}^{2}}-3{{y}^{2}}=6\]which is parallel to the line \[y-3x-4=0,\]is
A)
\[y=3x+8\]
done
clear
B)
\[y=3x-8\]
done
clear
C)
\[y=3x+2\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) If coefficient of correlation between two variables\[x\]and y is 0.32 and co variance is 8, variance of\[x\]is 25, then variance of y is
A)
36
done
clear
B)
25
done
clear
C)
64
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 104) Ten coins are tossed together, then the probability of getting at least 7 heads is
A)
\[\frac{63}{256}\]
done
clear
B)
\[\frac{121}{172}\]
done
clear
C)
\[\frac{113}{512}\]
done
clear
D)
\[\frac{11}{64}\]
done
clear
View Answer play_arrow
question_answer 105) If\[{{b}_{yx}}=0.03\]and\[{{b}_{xy}}=0.3,\]then the approximate value of r is
A)
0.003
done
clear
B)
0.095
done
clear
C)
0.3
done
clear
D)
\[-0.3\]
done
clear
View Answer play_arrow
question_answer 106)
Using Simpson's\[\frac{1}{3}\]rd rule, the value of\[\int_{1}^{5}{f(x)}dx\]for the following data is \[x\] 1 2 3 4 5 \[y\] 10 50 70 80 100
A)
140.88
done
clear
B)
256.66
done
clear
C)
160.26
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 107) The feasible region represented by\[{{x}_{1}}+{{x}_{2}}\le 1,\] \[-3{{x}_{1}}+{{x}_{2}}\ge ({{x}_{1}},{{x}_{2}}\ge 0)\]is
A)
a polygon
done
clear
B)
a singleton set
done
clear
C)
an empty set
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 108) The point on the curve\[{{x}^{2}}=2y\]which is at a minimum distance from the point (0,5), is
A)
(2,2), (-2,2)
done
clear
B)
\[(2\sqrt{2},4),(-2\sqrt{2},4)\]
done
clear
C)
\[(\sqrt{6},3),(-\sqrt{6},3)\]
done
clear
D)
\[(2\sqrt{3},6),(-2\sqrt{3},6)\]
done
clear
View Answer play_arrow
question_answer 109) The solution of the differential equation\[\left( \frac{dy}{dx} \right)\tan y=\sin (x+y)+\sin (x-y)\]is
A)
\[sec\text{ }x-1/2\text{ }tan\text{ }y=c\]
done
clear
B)
\[log\text{ }sin\text{ (}x+y)=c\]
done
clear
C)
\[sec\text{ }x+tan\text{ }y=c\]
done
clear
D)
\[sec\text{ }y+2\text{ }cos\text{ }x=c\]
done
clear
View Answer play_arrow
question_answer 110) \[\overrightarrow{A}.\{(\overrightarrow{B}+\overrightarrow{C})\times (\overrightarrow{A}+\overrightarrow{B}+\overrightarrow{C})\}\] is equal to
A)
\[[\overrightarrow{A}\,\overrightarrow{B}\,\overrightarrow{C}]\]
done
clear
B)
\[[\overrightarrow{B}\,\overrightarrow{A}\,\overrightarrow{C}]\]
done
clear
C)
0
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 111) If \[y+\frac{{{y}^{3}}}{3}+\frac{{{y}^{5}}}{5}+....\infty =2\left[ x+\frac{{{x}^{3}}}{3}+\frac{{{x}^{5}}}{5}+....\infty \right],\] then the value of y is
A)
\[\frac{x}{1-{{x}^{2}}}\]
done
clear
B)
\[\frac{2x}{1+{{x}^{2}}}\]
done
clear
C)
\[\frac{1-{{x}^{2}}}{2x}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 112) If\[x=\frac{1}{2}(\sqrt{3}+i),\]then\[{{x}^{3}}\]is equal to
A)
1
done
clear
B)
\[-1\]
done
clear
C)
2
done
clear
D)
\[-i\]
done
clear
View Answer play_arrow
question_answer 113) If the complex numbers\[sin\text{ }x+i\text{ }cos\text{ }2x\]and \[cos\,x-i\,sin2x\]are conjugate to each other, then the value of\[x\]is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{8}\]
done
clear
C)
\[\frac{3\pi }{4}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 114) \[\underset{x\to 2}{\mathop{\lim }}\,\frac{|x-2|}{x-2}\]is equal to
A)
\[-1\]
done
clear
B)
1
done
clear
C)
2
done
clear
D)
\[1-2\]
done
clear
View Answer play_arrow
question_answer 115) If \[\left| \begin{matrix} x & {{x}^{2}} & 1+{{x}^{3}} \\ y & {{y}^{2}} & 1+{{y}^{3}} \\ z & {{z}^{2}} & 1+{{z}^{3}} \\ \end{matrix} \right|=0,\]then
A)
\[z=xy\]
done
clear
B)
\[z=\frac{1}{xy}\]
done
clear
C)
\[z=-\frac{1}{xy}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) \[{{\cos }^{4}}\frac{\pi }{8}+{{\cos }^{4}}\frac{3\pi }{8}+{{\cos }^{4}}\frac{5\pi }{8}+{{\cos }^{4}}\frac{7\pi }{8}\]is equal to
A)
\[\frac{3}{2}\]
done
clear
B)
\[-\frac{2}{3}\]
done
clear
C)
\[-1\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 117) In\[\Delta ABC,\]if\[\tan \frac{A}{2}=\frac{5}{6}\]and\[\tan \frac{B}{2}=\frac{20}{37},\]then\[a+c\]is equal to
A)
b
done
clear
B)
2b
done
clear
C)
3b
done
clear
D)
4b
done
clear
View Answer play_arrow
question_answer 118) In the interval\[[0,2\pi ],\]the number of solutions of the equation\[tan\text{ }x+sec\text{ }x=2\text{ }cos\text{ }x\]is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 119) The joint equation of the bisectors of the angles between the lines represented by the equation\[a{{x}^{2}}+hxy+b{{y}^{2}}=0\]is
A)
\[\frac{{{x}^{2}}-{{y}^{2}}}{a-b}=\frac{2xy}{h}\]
done
clear
B)
\[\frac{{{x}^{2}}+{{y}^{2}}}{a+b}=\frac{xy}{2h}\]
done
clear
C)
\[\frac{{{x}^{2}}-{{y}^{2}}}{a-b}=\frac{xy}{h}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 120) The circle\[{{x}^{2}}+{{y}^{2}}-2cx-2cy+{{c}^{2}}=0\]touches both the axes and the line\[\frac{x}{3}+\frac{y}{4}=1\]. Its centre lies in the first quadrant, then the value of c is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 121) The angle between two diagonals of a cube is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[{{\cos }^{-1}}\left( \frac{1}{3} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{1}{\sqrt{3}} \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 122) If \[{{a}_{1}},{{a}_{2}},{{a}_{3}},....{{a}_{n}}\]are in AP when\[{{a}_{i}}>0\]for all\[i\], then the sum of the series \[\frac{1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{2}}}}+\frac{1}{\sqrt{{{a}_{2}}}+\sqrt{{{a}_{3}}}}+\frac{1}{\sqrt{{{a}_{3}}}+\sqrt{{{a}_{4}}}}\] \[+....+\frac{1}{\sqrt{{{a}_{n-1}}}+\sqrt{{{a}_{n}}}}\]is
A)
\[\frac{n+1}{\sqrt{{{a}_{1}}+{{a}_{n}}}}\]
done
clear
B)
\[\frac{n+1}{\sqrt{{{a}_{1}}}-\sqrt{{{a}_{n}}}}\]
done
clear
C)
\[\frac{n+1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{n}}}}\]
done
clear
D)
\[\frac{n-1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{n}}}}\]
done
clear
View Answer play_arrow
question_answer 123) Plane\[xy\]divides the line joining the points s (- 3, 4, - 8) and (5, - 6, 4) in the ratio
A)
\[2:3\]
done
clear
B)
\[2:1\]
done
clear
C)
\[4:5\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 124) The unit vectors which is perpendicular to both vectors\[3\hat{i}+\hat{j}+2\hat{k}\]and\[2\hat{i}-2\hat{j}+4\hat{k},\]is
A)
\[\frac{\hat{i}-\hat{j}-\hat{k}}{\sqrt{3}}\]
done
clear
B)
\[\frac{\hat{i}+\hat{j}+\hat{k}}{\sqrt{3}}\]
done
clear
C)
\[\frac{\hat{i}+\hat{j}-\hat{k}}{\sqrt{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) If\[y={{\tan }^{-1}}\frac{\sqrt{(1+{{x}^{2}})}+\sqrt{(1-{{x}^{2}})}}{\sqrt{(1+{{x}^{2}})}-\sqrt{(1-{{x}^{2}})}},\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{1}{\sqrt{1+{{x}^{2}}}}\]
done
clear
B)
\[-\frac{1}{2}\]
done
clear
C)
\[\frac{-x}{\sqrt{(x-{{x}^{4}})}}\]
done
clear
D)
\[\frac{x\sqrt{1+{{x}^{2}}}}{1-{{x}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 126) Vectors\[\overrightarrow{a},\text{ }\overrightarrow{b}\]and\[\overrightarrow{c}\]are in the same plane, which of the following is false?
A)
\[\overrightarrow{a}.(\overrightarrow{b}\times \overrightarrow{c})=0\]
done
clear
B)
\[\overrightarrow{a}\times (\overrightarrow{b}\times \overrightarrow{c})=0\]
done
clear
C)
\[[\overrightarrow{a}+\overrightarrow{b}\text{ }\overrightarrow{b}+\overrightarrow{c}\text{ }\overrightarrow{c}+\overrightarrow{a}]=0\]
done
clear
D)
\[\overrightarrow{a}=p\overrightarrow{b}+q\overrightarrow{c}\]
done
clear
View Answer play_arrow
question_answer 127) If\[[A]\ne 0\]and order of A is 21, then adj (adj A) is equal to
A)
\[|A{{|}^{n}}\]
done
clear
B)
\[|A{{|}^{2}}\]
done
clear
C)
\[|A{{|}^{n-1}}I\]
done
clear
D)
\[|A{{|}^{n-2}}A\]
done
clear
View Answer play_arrow
question_answer 128) \[\int{\frac{x+\sin x}{1+\cos x}}dx\]is equal to
A)
\[x\log (1+\cos x)+c\]
done
clear
B)
\[\frac{1}{x}\log (1+\cos x)+c\]
done
clear
C)
\[x\tan \frac{x}{2}+c\]
done
clear
D)
\[{{x}^{2}}{{\tan }^{-1}}\frac{x}{2}+c\]
done
clear
View Answer play_arrow
question_answer 129) The differential equation of the curve \[y=A{{e}^{x}}+B{{e}^{-x}}\]for different values of A and B, is
A)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}-2y=0\]
done
clear
B)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}=y\]
done
clear
C)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}=4y+3\]
done
clear
D)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}+y=0\]
done
clear
View Answer play_arrow
question_answer 130) The solution of\[\frac{dy}{dx}=\frac{{{y}^{2}}}{xy-{{x}^{2}}}\]is
A)
\[y=c{{e}^{x/y}}\]
done
clear
B)
\[y=c{{e}^{y/x}}+x\]
done
clear
C)
\[y=c{{e}^{y/x}}\]
done
clear
D)
\[xy=c{{e}^{y/x}}\]
done
clear
View Answer play_arrow
question_answer 131) If\[\underset{x\to a}{\mathop{\lim }}\,=\frac{{{a}^{x}}-{{x}^{a}}}{{{x}^{x}}-{{a}^{a}}}=-1,\]then
A)
\[a=1\]
done
clear
B)
\[a=0\]
done
clear
C)
\[a=e\]
done
clear
D)
\[a=\frac{1}{e}\]
done
clear
View Answer play_arrow
question_answer 132) Select four numbers from the numbers 1, 2, 3, 4, 5, 6, 7. The probability that the sum of these four numbers is less than 12, is
A)
\[\frac{3}{35}\]
done
clear
B)
\[\frac{4}{35}\]
done
clear
C)
\[\frac{2}{35}\]
done
clear
D)
\[\frac{1}{35}\]
done
clear
View Answer play_arrow
question_answer 133) Let A and B are two events, then the probability that exactly one event is occur, is
A)
\[P(A)+P(B)-2P(A\cap B)\]
done
clear
B)
\[P(A)+P(B)-P(A\cap B)\]
done
clear
C)
\[P(A)-P(B)\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 134) The solution of the equation \[x\cos x\left( \frac{dy}{dx} \right)+y(x\sin x+\cos x)=1\]is
A)
\[y=x\text{ }tan\text{ }x+sin\text{ }x+c\]
done
clear
B)
\[y=y\text{ }tan\text{ }x+c\]
done
clear
C)
\[yx\text{ }sec\text{ }x=tan\text{ }x+c\]
done
clear
D)
\[xy\text{ }cos\text{ }x=x+c\]
done
clear
View Answer play_arrow
question_answer 135) If equation\[({{a}^{2}}+4a+3){{x}^{2}}+({{a}^{2}}-a-2)x\] \[+a(a+1)=0\]have more than two roots, then the value of a is
A)
0
done
clear
B)
1
done
clear
C)
\[-1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 136) In an AP, the sum of the n, 2n and 3n terms are\[{{S}_{1}},{{S}_{2}}\]and\[{{S}_{3}}\]respectively, then
A)
\[{{S}_{2}}=3{{S}_{3}}-2{{S}_{1}}\]
done
clear
B)
\[{{S}_{3}}=4({{S}_{1}}+{{S}_{2}})\]
done
clear
C)
\[{{S}_{3}}=3({{S}_{2}}-{{S}_{1}})\]
done
clear
D)
\[{{S}_{3}}=2({{S}_{2}}+{{S}_{1}})\]
done
clear
View Answer play_arrow
question_answer 137) If\[^{n}{{C}_{r-1}}=36{{,}^{n}}{{C}_{r}}=84\]and\[^{n}{{C}_{r+1}}=126,\]then\[n\]is
A)
8
done
clear
B)
9
done
clear
C)
10
done
clear
D)
11
done
clear
View Answer play_arrow
question_answer 138) In a\[\Delta ABC,\]there are 3, 4 and 5 points on the sides AB, BC and CA respectively. The number of triangles formed by these points as vertices is
A)
201
done
clear
B)
120
done
clear
C)
205
done
clear
D)
435
done
clear
View Answer play_arrow
question_answer 139) In the expansion of \[1+\frac{a}{bx}1!+\frac{{{(a+bx)}^{2}}}{2!}\]\[+\frac{{{(a+bx)}^{3}}}{3!}+....\]the coefficient of\[{{x}^{n}}\]is
A)
\[\frac{{{e}^{a}}{{b}^{n}}}{n!}\]
done
clear
B)
\[\frac{{{(b.a)}^{n}}}{n!}\]
done
clear
C)
\[\frac{{{e}^{b}}.{{b}^{n}}}{(n-1)!}\]
done
clear
D)
\[\frac{{{a}^{n}}{{b}^{n-1}}}{n!}\]
done
clear
View Answer play_arrow
question_answer 140) \[\frac{{{C}_{0}}}{1}+\frac{{{C}_{2}}}{3}+\frac{{{C}_{4}}}{5}+\frac{{{C}_{6}}}{7}+....\]is equal to
A)
\[\frac{{{2}^{n-1}}}{n-1}\]
done
clear
B)
\[\frac{{{2}^{n+1}}}{n+3}\]
done
clear
C)
\[\frac{{{2}^{n}}}{n+1}\]
done
clear
D)
\[\frac{{{2}^{n-2}}}{n}\]
done
clear
View Answer play_arrow
question_answer 141) The equation of the parabola whose vertex is \[(2,-1)\]and focus\[(3,-1),\]is
A)
\[{{y}^{2}}-4x-2y+9=0\]
done
clear
B)
\[{{y}^{2}}+4x+2y-9=0\]
done
clear
C)
\[{{y}^{2}}-4x+2y+9=0\]
done
clear
D)
\[{{y}^{2}}+4x-2y+9=0\]
done
clear
View Answer play_arrow
question_answer 142) The equations of lines joining the origin to the point of intersection of the curves\[y=x+3\]and\[4{{x}^{2}}+4{{y}^{2}}=1,\]are
A)
\[36({{x}^{2}}+{{y}^{2}})={{(x-y)}^{2}}\]
done
clear
B)
\[12({{x}^{2}}+{{y}^{2}})={{(x+y)}^{2}}\]
done
clear
C)
\[9({{x}^{2}}+{{y}^{2}})=4{{(x-y)}^{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 143) The angle of elevation of a fighter jet plane from a point on the ground is\[60{}^\circ \]. After 10s the angle of elevation becomes\[30{}^\circ \]. If the speed of jet plane is 432 km/h, then the height of the jet plane from the ground is
A)
\[200\sqrt{3}m\]
done
clear
B)
\[400\sqrt{3}m\]
done
clear
C)
\[600\sqrt{3}m\]
done
clear
D)
\[800\sqrt{3}m\]
done
clear
View Answer play_arrow
question_answer 144) Which of the following is false?
A)
\[(AB)'=B'A'\]
done
clear
B)
\[{{(AB)}^{\theta }}={{B}^{\theta }}{{A}^{\theta }}\]
done
clear
C)
\[\overline{AB}=\overline{B}\,\,\overline{A}\]
done
clear
D)
\[{{(AB)}^{-1}}={{B}^{-1}}{{A}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 145) The equation of plane which is parallel to\[x-\]axis and passes through the line \[\frac{x-2}{2}=\frac{y-3}{3}=\frac{z-4}{5}\]is
A)
\[2x+3y+5z=1\]
done
clear
B)
\[2x-5y=4\]
done
clear
C)
\[5y-3z-3=0\]
done
clear
D)
\[3y+4z=0\]
done
clear
View Answer play_arrow
question_answer 146) A force\[\overrightarrow{F}=5\hat{i}+10\hat{j}+16\hat{k}\] acts at a point, whose position vector is\[2\hat{i}-7\hat{j}+10\hat{k}\]. The moment of F about the point\[-5\hat{i}+6\hat{j}-10\hat{k}\]is
A)
\[41\hat{i}-8\hat{j}+55\hat{k}\]
done
clear
B)
\[-408\hat{i}-12\hat{j}+135\hat{k}\]
done
clear
C)
\[-36\hat{i}+14\hat{j}-35\hat{k}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 147) The equations of tangent to the ellipse \[\frac{{{x}^{2}}}{{{a}^{2}}}+\frac{{{y}^{2}}}{{{b}^{2}}}=1\]which cut equal intercepts from the axes, are
A)
\[y=\sqrt{3}x\pm \sqrt{3{{a}^{2}}+{{b}^{2}}}\]
done
clear
B)
\[y=\pm x+\sqrt{{{a}^{2}}+{{b}^{2}}}\]
done
clear
C)
\[y=\sqrt{3x}\pm +\sqrt{{{a}^{2}}+3{{b}^{2}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 148) Square matrix A will be an orthogonal matrix, if
A)
\[A\overline{A}=I\]
done
clear
B)
\[AA'=I\]
done
clear
C)
\[A{{A}^{\theta }}=I\]
done
clear
D)
\[{{A}^{2}}=I\]
done
clear
View Answer play_arrow
question_answer 149) In the interval \[[0,\pi ],\] the function\[f(x)={{e}^{x}}\sin x\]satisfies the Rolle's theorem, the value of 'c' is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{3\pi }{4}\]
done
clear
C)
\[\frac{5\pi }{6}\]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 150) \[\int_{-a}^{a}{x\sqrt{({{a}^{2}}-{{x}^{2}})}}dx\]is equal to
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{3}\]
done
clear
C)
\[\frac{\pi }{8}\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
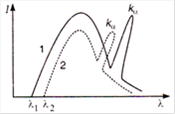 \[{{V}_{1}}\]and\[{{V}_{2}}\]and using different target materials of atomic numbers\[{{Z}_{1}}\]and\[{{Z}_{2}}\]is shown in the figure. Which of the following inequalities is true?
\[{{V}_{1}}\]and\[{{V}_{2}}\]and using different target materials of atomic numbers\[{{Z}_{1}}\]and\[{{Z}_{2}}\]is shown in the figure. Which of the following inequalities is true?
 is
is