question_answer 1) Four particles of masses m, 2m, 3m and 4m are arranged at the corners of a parallelogram with each side equal to a and one of the angle between two adjacent sides as\[60{}^\circ \]. The parallelogram lies in the \[x-y\] plane with mass m at the origin and 4m on the \[x-\]axis. The centre of mass of the arrangement will be located at
A)
\[\left( \frac{\sqrt{3}}{2}a,0.95a \right)\]
done
clear
B)
\[\left( 0.95a,\frac{\sqrt{3}}{4}a \right)\]
done
clear
C)
\[\left( \frac{3a}{4},\frac{a}{2} \right)\]
done
clear
D)
\[\left( \frac{a}{2},\frac{3a}{4} \right)\]
done
clear
View Answer play_arrow
question_answer 2) A 20 kg block is initially at rest on a rough horizontal surface. A horizontal force of 75 N is required to set the block in motion. After it is in motion, a horizontal force of 60 N is required to keep the block moving with constant speed. The coefficient of static friction is
A)
0.38
done
clear
B)
0.44
done
clear
C)
0.52
done
clear
D)
0.60
done
clear
View Answer play_arrow
question_answer 3) The total kinetic energy of a rolling sphere having translational velocity V is
A)
\[\frac{7}{10}M{{V}^{2}}\]
done
clear
B)
\[\frac{1}{2}M{{V}^{2}}\]
done
clear
C)
\[\frac{2}{5}M{{V}^{2}}\]
done
clear
D)
\[\frac{10}{7}M{{V}^{2}}\]
done
clear
View Answer play_arrow
question_answer 4) An elastic string has length\[\beta \]when subjected to 5 N tension. Its length is a when tension 4 N. When subjected to a tension of 9 N, its length will become
A)
\[9(\beta -\alpha )\]
done
clear
B)
\[5\alpha -4\beta \]
done
clear
C)
\[5\beta -4\alpha \]
done
clear
D)
\[\beta -\alpha \]
done
clear
View Answer play_arrow
question_answer 5) During a journey from earth to moon and back, the greatest energy required from the rocket of the space ship is to overcome
A)
the moons gravity at lunar landing
done
clear
B)
the moons gravity at lunar take off
done
clear
C)
the earths gravity at re-entry into earths atmosphere
done
clear
D)
the earths gravity at take off
done
clear
View Answer play_arrow
question_answer 6) A ball is rolled off the edge of a horizontal table at a speed of 4 m/second. It hits the ground after 0.4 second. Which statement given below is not true?
A)
it hits the ground at a horizontal distance 1.6 m from the edge of the table
done
clear
B)
the speed with which it hits the ground is 0.4m/second
done
clear
C)
height of the table is 0.8 m
done
clear
D)
it hits the ground at an angle of\[45{}^\circ \]to the horizontal
done
clear
View Answer play_arrow
question_answer 7) A body of mass M kg initially at rest explodes into three fragments having masses in the ratio\[3:1:1\]. The two equal mass fragments fly off at right angles to each other with equal speed of 60 m/s. The speed of the heavier fragment is
A)
\[30\sqrt{2}\,m/s\]
done
clear
B)
\[10\sqrt{2}\,m/s\]
done
clear
C)
\[20\sqrt{2}\,m/s\]
done
clear
D)
\[20\,m/s\]
done
clear
View Answer play_arrow
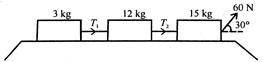
question_answer 8)
Three masses are connected (as Shown) on a horizontal frictionless surface and pulled by a force of 60 N. The tensions\[{{T}_{1}}\]and\[{{T}_{2}}\]are in the ratio
A)
\[1:1\]
done
clear
B)
\[1:5\]
done
clear
C)
\[1:4\]
done
clear
D)
\[4:5\]
done
clear
View Answer play_arrow
question_answer 9) A body is moved along a straight line by a machine which delivers constant power. The distance moved by the body in time\[t\]is proportional to
A)
\[t\]
done
clear
B)
\[{{t}^{1/2}}\]
done
clear
C)
\[{{t}^{5/2}}\]
done
clear
D)
\[{{t}^{3/2}}\]
done
clear
View Answer play_arrow
question_answer 10) If the speed of light [c], acceleration due to gravity [g] and pressure [P] are taken as the fundamental quantities, then the dimension of gravitational constant is
A)
\[{{c}^{2}}{{g}^{0}}{{p}^{-2}}\]
done
clear
B)
\[{{c}^{0}}{{g}^{2}}{{p}^{-1}}\]
done
clear
C)
\[c{{g}^{3}}{{p}^{-2}}\]
done
clear
D)
\[c{{g}^{0}}{{p}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 11) If the kinetic energy of one mole of an ideal gas is given as\[E=\frac{3}{2}RT\] (where 7? is the universal gas constant and T is the absolute temperature of the gas), the molar specific heat at constant pressure will be given as
A)
\[2.5\text{ }R\]
done
clear
B)
\[1.5\text{ }R\]
done
clear
C)
\[0.5\text{ }R\]
done
clear
D)
\[0.1\text{ }R\]
done
clear
View Answer play_arrow
question_answer 12) Which one of the following statement is true?
A)
for monoatomic gases \[\gamma =1.40\]
done
clear
B)
for diatomic gases \[{{C}_{p}}-{{C}_{V}}=2\text{ }cal\]
done
clear
C)
for polyatomic gases \[\gamma =1.67\]
done
clear
D)
for all the gases \[{{C}_{p}}=4R\]
done
clear
View Answer play_arrow
question_answer 13) A Carnot engine working between 300 K and 600 K has a work output of 800 joules per cycle. The amount of heat energy supplied from the source to the engine in each cycle is
A)
6400 joule
done
clear
B)
1600 joule
done
clear
C)
3200 joule
done
clear
D)
800 joule
done
clear
View Answer play_arrow
question_answer 14) Oxygen and hydrogen gases are at the same temperature and pressure. The oxygen molecule has 16 times the mass of hydrogen molecule. The ratio of the root mean square velocity of oxygen molecule to that of hydrogen molecule will be
A)
16
done
clear
B)
4
done
clear
C)
2
done
clear
D)
¼
done
clear
View Answer play_arrow
question_answer 15) A thin metal disc of radius r floats on water surface and bends the surface downwards along the perimeter making an angle\[\theta \]with verticle edge of the disc. If the disc displaces a weight of water W and surface tension of water is T, then the weight of metal disc is
A)
\[2\pi rT+W\]
done
clear
B)
\[2\pi rT\cos \theta -W\]
done
clear
C)
\[2\pi rT\cos \theta +W\]
done
clear
D)
\[W-2\pi rT\cos \theta \]
done
clear
View Answer play_arrow
question_answer 16) A block of mass is suspended from a spring balance. When the block is in air the balance reads 120 N. When it is immersed in water the reading changes to 80 N. The relative density of the material of the block is
A)
6
done
clear
B)
\[\frac{6}{2}\]
done
clear
C)
\[\frac{6}{3}\]
done
clear
D)
\[\frac{6}{4}\]
done
clear
View Answer play_arrow
question_answer 17) A 10 cm long wire is placed horizontally on the surface of water and is gently pulled up with a force of \[2\times {{10}^{-2}}N\]to keep the wire in equilibrium. The surface tension, in\[N{{m}^{-1}}\]of water is
A)
0.1
done
clear
B)
0.2
done
clear
C)
0.001
done
clear
D)
0.002
done
clear
View Answer play_arrow
question_answer 18) Radius of gyration of uniform thin rod of length about an axis passing normally through its centre of mass is
A)
\[\frac{L}{\sqrt{12}}\]
done
clear
B)
\[\frac{L}{12}\]
done
clear
C)
\[\sqrt{12}L\]
done
clear
D)
\[12L\]
done
clear
View Answer play_arrow
question_answer 19) An artificial satellite is placed into a circular orbit around earth at such a height that it always remains about a definite place on the surface of earth. Its height from the surface of earth is
A)
6400 km
done
clear
B)
4800 km
done
clear
C)
32000km
done
clear
D)
36000km
done
clear
View Answer play_arrow
question_answer 20) A pendulum bob of mass 50 gm is suspended from the ceiling of an elevator. The tension in the string if the elevator goes up with uniform velocity is approximately
A)
0.30 N
done
clear
B)
0.40 N
done
clear
C)
0.42 N
done
clear
D)
0.50 N
done
clear
View Answer play_arrow
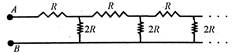
question_answer 21)
An infinite ladder network is arranged with resistances\[R\]and\[2R\]as shown. The effective resistance between terminals A and B is
A)
\[\infty \]
done
clear
B)
\[R\]
done
clear
C)
\[2R\]
done
clear
D)
\[3R\]
done
clear
View Answer play_arrow
question_answer 22) A wire of resistance\[1\,\Omega \]is stretched to double its length. The resistance of the wire will become
A)
\[1\,\Omega \]
done
clear
B)
\[\frac{1}{4}\,\Omega \]
done
clear
C)
\[2\,\Omega \]
done
clear
D)
\[4\,\Omega \]
done
clear
View Answer play_arrow
question_answer 23) The electric field in a region is radially outward with magnitude\[E=Ar\]. The charge contained in a sphere of radius\[{{r}_{0}}\]centered at the origin is
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}Ar_{0}^{3}\]
done
clear
B)
\[4\pi {{\varepsilon }_{0}}\,Ar_{0}^{3}\]
done
clear
C)
\[\frac{4\pi {{\varepsilon }_{0}}A}{r_{0}^{3}}\]
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{A}{r_{0}^{3}}\]
done
clear
View Answer play_arrow
question_answer 24) If a slab of insulating material\[4\times {{10}^{-3}}\]m thick is introduced between the plates of a parallel plate capacitor, the separation between plates has to be increased by\[3.5\times {{10}^{-3}}\]m to restore the capacity to original value. The dielectric constant of the material will be
A)
6
done
clear
B)
8
done
clear
C)
10
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 25) What is the potential energy of the equal positive point charges of\[1\,\mu C\]each held 1 m apart in air?
A)
\[9\times {{10}^{-3}}J\]
done
clear
B)
\[9\times {{10}^{-3}}eV\]
done
clear
C)
\[1J\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 26) A whistle of frequency 500 Hz tied to the end of a string of length 1.2 m revolves at 400 revolutions per minute. If the velocity of sound is 340 m/sec, a listener standing some distance away in the plane of rotation of the whistle hears the frequencies in the range
A)
436 to 586 Hz
done
clear
B)
426 to 574 Hz
done
clear
C)
436 to 574 Hz
done
clear
D)
426 to 586 Hz
done
clear
View Answer play_arrow
question_answer 27) A stationary wave is expressed as \[y=4\sin \left( \frac{\pi x}{3} \right)\cos (40\pi t)\] where\[x\] and y are measured in cm and time t in second. The separation between consecutive nodes is
A)
1.5 cm
done
clear
B)
6 cm
done
clear
C)
12 cm
done
clear
D)
3 cm
done
clear
View Answer play_arrow
question_answer 28) A\[1.00\times {{10}^{-20}}kg\]particle is vibrating with simple harmonic motion with a period of\[1.00\times {{10}^{-5}}\]s and a maximum speed of\[1.00\times {{10}^{3}}m/s\]. The maximum displacement of the particle is
A)
1.59mm
done
clear
B)
1.00m
done
clear
C)
10 m
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 29) A Simple harmonic wave is represented by the relation\[y=a\sin 2\pi \left( pt-\frac{x}{\lambda } \right)\]where a and y are measured in cm and time\[t\]in seconds. The maximum particle velocity is five times the wave velocity. The wavelength of the wave is
A)
\[\frac{2\pi a}{\sqrt{5}}cm\]
done
clear
B)
\[\frac{2\pi a}{5}cm\]
done
clear
C)
\[\frac{\pi a}{5}cm\]
done
clear
D)
\[2\sqrt{5}\pi a\,cm\]
done
clear
View Answer play_arrow
question_answer 30) A slab of thickness\[{{d}_{1}}\]and coefficient of thermal conductivity\[{{K}_{1}}\]. is placed in contact with another slab of thickness\[{{d}_{2}}\]and coefficient of thermal conductivity\[{{K}_{2}}\]. In steady state, the conductivity of composite slab will be
A)
\[\frac{{{d}_{1}}+{{d}_{2}}}{{{K}_{1}}+{{K}_{2}}}\]
done
clear
B)
\[\frac{{{K}_{1}}{{K}_{2}}}{{{K}_{1}}{{d}_{2}}+{{K}_{2}}{{d}_{1}}}\]
done
clear
C)
\[\frac{{{K}_{1}}{{d}_{1}}+{{K}_{2}}{{d}_{2}}}{{{d}_{1}}+{{d}_{2}}}\]
done
clear
D)
\[\frac{{{d}_{1}}+{{d}_{2}}}{\left( \frac{{{d}_{1}}}{{{K}_{1}}}+\frac{{{d}_{2}}}{{{K}_{2}}} \right)}\]
done
clear
View Answer play_arrow
question_answer 31) What is the frequency of radio waves corresponding to 10 m wavelength?
A)
\[3\times {{10}^{9}}Hz\]
done
clear
B)
\[3.3\times {{10}^{-8}}Hz\]
done
clear
C)
\[3\times {{10}^{7}}Hz\]
done
clear
D)
\[3.4\times {{10}^{-7}}Hz\]
done
clear
View Answer play_arrow
question_answer 32) A monochromatic light beam of wavelength 2537 A is falling normally on a metal plate. If the maximum velocity of emitted photoelectron is \[1.6\times {{10}^{5}}m/sec\] and a constant magnetic field of strength \[{{10}^{-4}}\] \[weber/{{m}^{2}}\]is applied parallel to metal surface, the radius of the largest circular path of photoelectrons is
A)
0.61 m
done
clear
B)
0.61 cm
done
clear
C)
\[6.1\times {{10}^{-4}}m\]
done
clear
D)
\[6.1\times {{10}^{-5}}m\]
done
clear
View Answer play_arrow
question_answer 33) Suppose electromagnetic waves having speed \[2.5\times {{10}^{8}}\]m/sec are travelling in a medium of relative permeability 0.8. The relative permitivity is
A)
1.0
done
clear
B)
1.8
done
clear
C)
2.5
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 34) The effective value of A.C. in a circuit is 10 A. The peak value of current is
A)
5 A
done
clear
B)
0.707 A
done
clear
C)
10 A
done
clear
D)
14.14 A
done
clear
View Answer play_arrow
question_answer 35) An LC circuit contains a 40 mH inductor and a \[25\,\mu F\]capacitor. The resistance of the circuit is negligible. The energy stored in the circuit is completely magnetic at times (in milliseconds). The time is measured from the instant the circuit is closed
A)
0, 3.14, 6.28
done
clear
B)
0, 1.57, 4.71
done
clear
C)
1.57, 4.71, 7.85
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 36) A cyclotron is accelerating proton, where the applied magnetic field is 2 T, the potential gap is 100 kV then how much turn the proton has to move between the dees to acquire a kinetic energy of 20 MeV
A)
100
done
clear
B)
150
done
clear
C)
200
done
clear
D)
300
done
clear
View Answer play_arrow
question_answer 37) The magnetic induction in the region between the pole faces of an electromagnet is 0.7 weber/\[{{m}^{2}}\]. The induced e.m.f. in a straight conductor 10 cm long, perpendicular to B and moving perpendicular both to magnetic induction and its own length with a velocity 2 m/sec is
A)
0.08 V
done
clear
B)
0.14V
done
clear
C)
0.35 V
done
clear
D)
0.07 V
done
clear
View Answer play_arrow
question_answer 38) A circular coil of diameter 7cm has 24 turns of wire carrying current of 0.75A. The magnetic moment of the coil is
A)
\[6.9\times {{10}^{-2}}A{{m}^{2}}\]
done
clear
B)
\[2.3\times {{10}^{-2}}A{{m}^{2}}\]
done
clear
C)
\[{{10}^{-2}}A{{m}^{2}}\]
done
clear
D)
\[{{10}^{-3}}A{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 39) A certain current passing through a copper voltameter deposits 0.002 kg of copper on cathode plate in 100 minutes. If there are\[{{10}^{25}}\] copper atoms in 1kg of copper, the electric charge delivered to cathode by copper ions\[(C{{u}^{2+}})\]per second will be
A)
1.06 C
done
clear
B)
2.13 C
done
clear
C)
0.53 C
done
clear
D)
0.71 C
done
clear
View Answer play_arrow
question_answer 40) A current\[i\]passes through a wire of length\[l\], radius of cross-section r, and resistivity \[\rho \]. The rate of heat generation is
A)
\[\frac{{{i}^{2}}l\rho }{\pi {{r}^{2}}}\]
done
clear
B)
\[{{i}^{2}}{{\left( \frac{l\rho }{\pi {{r}^{2}}} \right)}^{2}}\]
done
clear
C)
\[{{i}^{2}}l\rho /r\]
done
clear
D)
\[il\rho /r\]
done
clear
View Answer play_arrow
question_answer 41) The star Sirius is emitting radiation with the radiant intensity (energy emitted per unit area per second) of\[1.2\times {{10}^{7}}W/{{m}^{2}}\]the colour of this star will be
A)
blue
done
clear
B)
yellow
done
clear
C)
green
done
clear
D)
red
done
clear
View Answer play_arrow
question_answer 42) Transistors provide good power amplification when they are used in
A)
common emitter configuration
done
clear
B)
common base configuration
done
clear
C)
common collector configuration
done
clear
D)
none of the above
done
clear
View Answer play_arrow
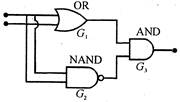
question_answer 43)
The following combination of gates is equivalent to which of the following gate
A)
XOR
done
clear
B)
OR
done
clear
C)
NAND
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 44) When a hydrogen atom is raised from the ground state to an excited state
A)
both kinetic energy and potential energy increase
done
clear
B)
both kinetic energy and potential energy decrease
done
clear
C)
potential energy increases, kinetic energy decreases
done
clear
D)
potential energy decreases, kinetic energy increases
done
clear
View Answer play_arrow
question_answer 45) A freshly prepared radioactive substance of half-life half an hour emits radiation of intensity thirty two times the permissible safe limit. The minimum time after which it would be safe to work with the substance is
A)
3 hours
done
clear
B)
6 hours
done
clear
C)
2 hours
done
clear
D)
8 hours
done
clear
View Answer play_arrow
question_answer 46) Consider the following two statements [A] Energy spectrum of a particles emitted in radioactive decay is discrete [B] Energy spectrum of P particles emitted in radioactive decay is continuous
A)
only A is correct
done
clear
B)
only B is correct
done
clear
C)
A is correct but B is wrong
done
clear
D)
both A and B are correct
done
clear
View Answer play_arrow
question_answer 47) An oil drop having charge 2e is kept stationary between two parallel horizontal plates 2.0 cm apart when a potential difference of 12000 volts is applied between them. If the density of oil is\[900\text{ }kg/{{m}^{3}}\], the radius of the drop will be
A)
\[2.0\times {{10}^{-6}}m\]
done
clear
B)
\[1.7\times {{10}^{-6}}m\]
done
clear
C)
\[1.4\times {{10}^{-6}}m\]
done
clear
D)
\[1.1\times {{10}^{-6}}m\]
done
clear
View Answer play_arrow
question_answer 48) Energy required to remove an electron from aluminium surface is 4.2 eV. If light of wavelength 2000 A falls on the surface, the velocity of the fastest electron ejected from the surface will be
A)
\[8.4\times {{10}^{5}}\]m/second
done
clear
B)
\[7.4\times {{10}^{5}}\]m/second
done
clear
C)
\[6.4\times {{10}^{5}}\]m/second
done
clear
D)
\[8.4\times {{10}^{6}}\]m/second
done
clear
View Answer play_arrow
question_answer 49) A convex lens is used to form real image of an object on a screen. It is observed that even when the positions of the object and the screen are fixed there are two positions of the lens to form real images. If the heights of the images are 4 cm and 9cm respectively, the height of the objective is;
A)
2.25 cm
done
clear
B)
6.00 cm
done
clear
C)
6.50 cm
done
clear
D)
36.00 cm
done
clear
View Answer play_arrow
question_answer 50) A compound microscope is 20 cm long and the power of its eye-piece is 20 D. If the objective is bi-convex (radius of curvature of either surface 2 cm) and made of a material of = 1.5, the magnifying power of the microscope is
A)
54
done
clear
B)
60
done
clear
C)
13.5
done
clear
D)
41.5
done
clear
View Answer play_arrow
question_answer 51) The internal energy of a system is the sum of
A)
vibrational energy and electronic energy
done
clear
B)
vibrational energy and rotational energy
done
clear
C)
vibrational energy, rotational energy, electronic energy and translational energy
done
clear
D)
rotational energy and electronic energy
done
clear
View Answer play_arrow
question_answer 52) The maximum number of permissible rotational orientations of the Is electron of lithium atom in ground state is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 53) Which of the following is the correct bond order for\[N_{2}^{2-}\]ion?
A)
2.5
done
clear
B)
2.0
done
clear
C)
3.0
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 54) Of the following iso-electronic ions, the one which has the lowest ionization potential is
A)
\[N{{a}^{+}}\]
done
clear
B)
\[M{{g}^{++}}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
\[{{O}^{--}}\]
done
clear
View Answer play_arrow
question_answer 55) What is the effect of more electronegative atom on the strength of ionic bond?
A)
decreases
done
clear
B)
increases
done
clear
C)
remains same
done
clear
D)
any one of these
done
clear
View Answer play_arrow
question_answer 56) If we consider the ammonia molecule to be \[s{{p}^{3}}\] hybridized, the lone pair will occupy
A)
an s-orbital
done
clear
B)
a p-orbital
done
clear
C)
a\[s{{p}^{2}}-\]hybridized orbital
done
clear
D)
a\[s{{p}^{3}}-\]hybridized orbital
done
clear
View Answer play_arrow
question_answer 57) Rate constants of some reactions are given below. Predict which one indicates third order of reaction?
A)
\[k=8.7\times {{10}^{-3}}mo{{l}^{-1}}L{{s}^{-1}}\]
done
clear
B)
\[k=6.5\times {{10}^{-4}}mol{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[k=5.3\times {{10}^{-5}}{{s}^{-1}}\]
done
clear
D)
\[k=2.52\times {{10}^{-32}}mo{{l}^{-2}}{{L}^{2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 58) A zinc rod is placed in 0.095 M solution of zinc sulphate at 298 K, the potential of the electrode at this temperature is[\[E_{Zn}^{o}=-0.76\]volts]
A)
\[-0.79\]volts
done
clear
B)
\[-0.69\]volts
done
clear
C)
\[-0.59\]volts
done
clear
D)
\[-0.49\]volts
done
clear
View Answer play_arrow
question_answer 59) Which of the following hydrogen bonds are strongest in vapour phase?
A)
\[HF---HF\]
done
clear
B)
\[HF---HCl\]
done
clear
C)
\[HCl---HCl\]
done
clear
D)
\[HF---HI\]
done
clear
View Answer play_arrow
question_answer 60) A primary alcohol,\[{{C}_{3}}{{H}_{8}}O\] on heating with sulphuric acid undergo dehydration to give an alkene, B.B when reacted with\[HCl\]gave C, which on treatment with aqueous KOH gives compound \[D,{{C}_{3}}{{H}_{8}}O.A\]and D are
A)
functional isomers
done
clear
B)
position isomers
done
clear
C)
chain isomers
done
clear
D)
stereo isomers
done
clear
View Answer play_arrow
question_answer 61) In the series of bivalent hydrides of sixteenth group elements
A)
volatility increases from\[{{H}_{2}}O\]to\[{{H}_{2}}S\]and then decreases
done
clear
B)
volatility increases from\[{{H}_{2}}O\]to \[{{H}_{2}}Se\]
done
clear
C)
volatility decreases from\[{{H}_{2}}O\]to\[{{H}_{2}}Se\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 62) Of the aliphatic compound carrying the following functional groups the one which cannot exhibit position isomerism is
A)
carbon-carbon double bond
done
clear
B)
carbon-carbon triple bond
done
clear
C)
keto group
done
clear
D)
carboxylic acid group
done
clear
View Answer play_arrow
question_answer 63) When ethanol is subjected to dehydration in presence of\[{{H}_{2}}S{{O}_{4}},\]the intermediate formed is a
A)
carbonium ion
done
clear
B)
carbanion
done
clear
C)
radical
done
clear
D)
carbine
done
clear
View Answer play_arrow
question_answer 64) Which of the following can act as an acid and as a base?
A)
\[HCO_{3}^{-}\]
done
clear
B)
\[{{H}_{2}}PO_{4}^{-}\]
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 65) \[200\text{ }c{{m}^{3}}\]of an aqueous solution of a protein contains 1.26 g. of protein. The O.P. of such a solution at 300 K is found to be\[2.57\times {{10}^{-3}}\]atm. Molar mass of protein is
A)
\[60287\text{ }g.mo{{l}^{-1}}\]
done
clear
B)
\[30242.\text{ }g.mo{{l}^{-1}}\]
done
clear
C)
\[40404\text{ }g.\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[50404\text{ }g.\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 66) What is the magnetic moment for\[M{{n}^{2+}}\]ion in low spin state?
A)
5.90 BM
done
clear
B)
8.90 BM
done
clear
C)
2.8 BM
done
clear
D)
1.73 BM
done
clear
View Answer play_arrow
question_answer 67) On mole of a gas absorbs 300 J of heat at constant volume and its temperature is raised from 30 to\[35{}^\circ C\]. The value of\[\Delta E\]is
A)
300 joules
done
clear
B)
0.1 joules
done
clear
C)
150 joules
done
clear
D)
200 joules
done
clear
View Answer play_arrow
question_answer 68) In which of the following conditions a chemical reaction can not occur?
A)
\[\Delta H\] and\[\Delta S\]increase and\[T\Delta S>\Delta H\]
done
clear
B)
\[\Delta H\]and\[\Delta S\]decrease and \[\Delta H>T\Delta S\]
done
clear
C)
\[\Delta H\]increases and\[\Delta S\]increases
done
clear
D)
\[\Delta H\]decreases and\[\Delta S\]increases
done
clear
View Answer play_arrow
question_answer 69) If the melting point of ice is\[0{}^\circ C\]and latent heat of fusion is\[5.46\text{ }kJ\text{ }mo{{l}^{-1}}\], the entropy change for the conversion of 18 g of ice to water at one atmosphere is
A)
\[5.46\text{ }J{{K}^{-1}}\]
done
clear
B)
\[20\text{ }J{{K}^{-1}}\]
done
clear
C)
\[50\text{ }J{{K}^{-1}}\]
done
clear
D)
\[\text{360 }J{{K}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 70) Calculate the uncertainty in the velocity of a cricket ball of 100 g if the uncertainty in its position is\[1.65\text{ }\overset{o}{\mathop{\text{A}}}\,\].
A)
\[1.657\pi \times {{10}^{-43}}m/s\]
done
clear
B)
\[\frac{6.6}{\pi }\times {{10}^{-45}}m/s\]
done
clear
C)
\[\frac{{{10}^{-23}}}{\pi }m/s\]
done
clear
D)
\[\frac{{{10}^{-26}}}{\pi }m/s\]
done
clear
View Answer play_arrow
question_answer 71) The correct order of basicity of amines in water is
A)
\[C{{H}_{3}}N{{H}_{2}}>{{(C{{H}_{3}})}_{2}}NH>{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}N>{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}NH>{{(C{{H}_{3}})}_{3}}N>C{{H}_{3}}N{{H}_{2}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 72) Solubility of lead chloride is K. Then the number of moles of \[C{{l}^{-}}\]ionsin 1000 ml of saturated lead chloride solution will be
A)
\[{{\left( \frac{K}{4} \right)}^{1/3}}\]
done
clear
B)
\[{{(2K)}^{1/3}}\]
done
clear
C)
\[{{K}^{1/2}}\]
done
clear
D)
\[\frac{K}{2}\]
done
clear
View Answer play_arrow
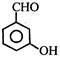
question_answer 73) The compounds that will undergo Cannizzaro reaction are 1. \[C{{H}_{3}}CHO\] 2. \[{{(C{{H}_{3}})}_{3}}CCHO\] 3. \[PhCHO\] 4. \[C{{H}_{3}}COC{{H}_{3}}\]
A)
1 and 2
done
clear
B)
1 and 3
done
clear
C)
2 and 3
done
clear
D)
2 and 4
done
clear
View Answer play_arrow
question_answer 74) The freezing point of a 0.01 m aqueous glucose solution at 1 atmosphere is\[-0.18{}^\circ C\]. To it, an addition of equal volume of 0.002 m glucose solution will produce a solution with freezing point of nearly
A)
\[-0.036{}^\circ C\]
done
clear
B)
\[-0.108{}^\circ C\]
done
clear
C)
\[-0.216{}^\circ C\]
done
clear
D)
\[-0.422{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 75) 60 J of heat flows out from 600 g. of\[{{H}_{2}}O\]at \[30{}^\circ C\]into the surroundings at\[25{}^\circ C\]. The total entropy change in the universe due to this process is
A)
\[0.1\text{ }J{{K}^{-1}}\]
done
clear
B)
\[0.5\text{ }J{{K}^{-1}}\]
done
clear
C)
\[0.9J{{K}^{-1}}\]
done
clear
D)
\[1.0\text{ }J{{K}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following is a better electron pair donor?
A)
\[P{{(C{{H}_{3}})}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[N{{(C{{H}_{3}})}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) The compounds that will give an isomer of 2,2- dimethylpropane on catalytic hydrogenation are (1) \[C{{H}_{3}}CH=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] (2) \[C{{H}_{3}}CH=CHC{{H}_{3}}\] (3) \[C{{H}_{3}}CH=CHC{{H}_{2}}C{{H}_{3}}\] (4) \[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
A)
1 and 4
done
clear
B)
2 and 4
done
clear
C)
1 and 3
done
clear
D)
1 and 2
done
clear
View Answer play_arrow
question_answer 78) What is the correct mode of hybridization of the central atom in the following compounds?
A)
\[NO_{2}^{+}\] - \[s{{p}^{2}}\] \[S{{F}_{4}}\] - \[s{{p}^{3}}\] \[PF_{6}^{-}\] - \[{{d}^{2}}s{{p}^{3}}\]
done
clear
B)
\[NO_{2}^{+}\] - \[s{{p}^{3}}\] \[S{{F}_{4}}\] - \[s{{p}^{3}}{{d}^{2}}\] \[PF_{6}^{-}\] - \[s{{p}^{3}}{{d}^{2}}\]
done
clear
C)
\[NO_{2}^{+}\] - \[sp\] \[S{{F}_{4}}\] - \[s{{p}^{3}}d\] \[PF_{6}^{-}\] - \[s{{p}^{3}}{{d}^{2}}\]
done
clear
D)
\[NO_{2}^{+}\] - \[sp\] \[S{{F}_{4}}\] - \[s{{p}^{2}}\] \[PF_{6}^{-}\] - \[s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following statements is correct?
A)
\[S{{F}_{4}}\]is polar and nonreactive
done
clear
B)
\[S{{F}_{6}}\]is nonpolar and very reactive
done
clear
C)
\[S{{F}_{6}}\]is a strong fluorinating agent
done
clear
D)
\[S{{F}_{4}}\]is prepared by fluorinating\[SC{{l}_{2}}\]with\[NaF\]
done
clear
View Answer play_arrow
question_answer 80) An ionic compound\[XY\]has a structure of ZnS type. If the radius of\[{{X}^{+}}\]is 22.5 pm. The ideal radius of\[Y-\]will be
A)
100 pm
done
clear
B)
50 pm
done
clear
C)
20 pm
done
clear
D)
30 pm
done
clear
View Answer play_arrow
question_answer 81) The pH of a soft drink is 3.82. The hydrogen ion concentration of the drink is (Antilog 0.18=1.5)
A)
\[1.96\times {{10}^{-2}}mole\text{ }li{{t}^{-1}}\]
done
clear
B)
\[1.96\times {{10}^{-3}}mole\text{ }li{{t}^{-1}}\]
done
clear
C)
\[1.5\times {{10}^{-4}}mole\text{ }li{{t}^{-1}}\]
done
clear
D)
\[1.5\times {{10}^{-3}}mole\text{ }li{{t}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 82) If an amide is treated with\[{{P}_{2}}{{O}_{5}}\]the likely product is an
A)
acid
done
clear
B)
alkyl cyanide
done
clear
C)
amine
done
clear
D)
acid anhydride
done
clear
View Answer play_arrow
question_answer 83) The polymer used for making contact lenses for eyes is
A)
poly methyl methacrylate
done
clear
B)
polyethylene
done
clear
C)
polyethyl acrylate
done
clear
D)
nylon 6
done
clear
View Answer play_arrow
question_answer 84) Silver sulphide dissolves in a solution of sodium cyanide to form the complex
A)
\[Na[Ag{{(CN)}_{2}}]\]
done
clear
B)
\[N{{a}_{3}}[Ag{{(CN)}_{4}}]\]
done
clear
C)
\[N{{a}_{5}}[Ag{{(CN)}_{6}}]\]
done
clear
D)
\[N{{a}_{2}}[Ag{{(CN)}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 85) The compound that can be formed by aldol condensation of acetaldehyde is
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-COOH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
View Answer play_arrow
question_answer 86) Phenol is less acidic than
A)
acetylene
done
clear
B)
para methoxy phenol
done
clear
C)
ethanol
done
clear
D)
para nitro phenol
done
clear
View Answer play_arrow
question_answer 87) In a\[{{H}_{2}}{{O}_{2}}\]fuel cell, 67.2 litre of\[{{H}_{2}}\]at STP react in 10 minutes as per following reaction- Anode \[{{H}_{2}}+2O{{H}^{-}}\to 2{{H}_{2}}O+2{{e}^{-}}\] Cathode \[{{O}_{2}}+2{{H}_{2}}O+2{{e}^{-}}\to 4O{{H}^{-}}\] The average current produced by the fuel cell will be
A)
5 milliampere
done
clear
B)
10 milliampere
done
clear
C)
482.7 ampere
done
clear
D)
965.4 ampere
done
clear
View Answer play_arrow
question_answer 88) The element of atomic number 11 is highly reactive, which of the following elements will be most likely to be reactive metal?
A)
Elementwithat.no. 17
done
clear
B)
Element with at. no. 24
done
clear
C)
Element with at. no. 37
done
clear
D)
Element with at. no. 54
done
clear
View Answer play_arrow
question_answer 89) What type of hybridization is involved in \[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
A)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
B)
\[ds{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}{{d}^{3}}\]
done
clear
D)
\[ds{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 90) Molar conductivity of a solution of an electrolyte\[A{{B}_{2}}\]is\[160\text{ }oh{{m}^{-1}}c{{m}^{2}}mo{{l}^{-1}}\]. If it ionizes as\[A{{B}_{2}}\to {{A}^{+2}}+2{{B}^{-1}}\]. Its equivalent conductivity will be
A)
\[150\text{ }oh{{m}^{-1}}c{{m}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[\text{75 }oh{{m}^{-1}}c{{m}^{-1}}mo{{l}^{-1}}\]
done
clear
C)
\[\text{80 }oh{{m}^{-1}}c{{m}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 91) In\[{{S}_{N}}2\]reactions the sequence of bond breaking and bond formation is as follows
A)
bond breaking is followed by formation
done
clear
B)
bond formation is followed by breaking
done
clear
C)
bond breaking and formation are simultaneous
done
clear
D)
bond breaking and formation take place randomly
done
clear
View Answer play_arrow
question_answer 92) Which of the following on nuclear bromination can yield only one monobromo derivative?
A)
ortho xylene
done
clear
B)
meta xylene
done
clear
C)
para xylene
done
clear
D)
toluene
done
clear
View Answer play_arrow
question_answer 93) Which of the following shows highest magnetic moment?
A)
\[{{V}^{3+}}\]
done
clear
B)
\[C{{r}^{3+}}\]
done
clear
C)
\[M{{n}^{2+}}\]
done
clear
D)
\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 94) Which of the following can exhibit keto-enol tautomerism?
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
B)
\[CC{{l}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
C)
\[H-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}C-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
View Answer play_arrow
question_answer 95) CP-LCN is known as acetonitrile because
A)
it contains an aceto group
done
clear
B)
on hydrolysis it gives acetic acid
done
clear
C)
acetic acid is converted to it by direct reaction with HCN
done
clear
D)
acetyl chloride on reaction with HCN gives the compound
done
clear
View Answer play_arrow
question_answer 96) In the following four compounds, the one which is a functional isomer of the set of other three is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 97) Diethyl ether is isomeric with
A)
primary alcohol
done
clear
B)
secondary alcohol
done
clear
C)
tert. alcohol
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 98) The same quantity of electricity was passed through salt solutions of silver (at. wt. = 107.9) and gold (at. wt. = 197). If 1.439 g of Ag and 0.876 g of Au were deposited, the oxidation state of Au in the salt is
A)
zero
done
clear
B)
+2
done
clear
C)
+3
done
clear
D)
+1
done
clear
View Answer play_arrow
question_answer 99) \[{{H}_{2}}S\]acts only as a reducing agent while\[S{{O}_{2}},\]can act both as a reducing and oxidizing agent because
A)
S in\[{{H}_{2}}S\]has -2 oxidation state
done
clear
B)
S in\[S{{O}_{2}}\]has oxidation state\[+4\]
done
clear
C)
hydrogen in\[{{H}_{2}}S\]is more +ve than oxygen in\[S{{O}_{2}}\]
done
clear
D)
oxygen is more -ve in\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) In the equilibrium \[{{A}^{-}}+{{H}_{2}}O\rightleftharpoons HA+O{{H}^{-}}\] \[({{K}_{a}}=1.0\times {{10}^{-5}})\] The degree of hydrolysis of 0.001 M solution of the salt is
A)
\[{{10}^{-3}}\]
done
clear
B)
\[{{10}^{-4}}\]
done
clear
C)
\[{{10}^{-5}}\]
done
clear
D)
\[{{10}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 101) A situation where one population is benefitted while the other unaffected is referred as
A)
commensalism
done
clear
B)
parasitism
done
clear
C)
amensalism
done
clear
D)
symphily.
done
clear
View Answer play_arrow
question_answer 102) Which of the following is incorrect?
A)
iodine is needed for thyroxine formation
done
clear
B)
calcium regulates the excitibility of nerve fibres ag
done
clear
C)
potassium plays an important role in the regulation of acid-base balance in cell
done
clear
D)
phosphorous helps to maintain the osmotic pressure of the body fluids.
done
clear
View Answer play_arrow
question_answer 103) The first antibody which gets formed in response to an antigen is
A)
\[IgM\]
done
clear
B)
\[IgG\]
done
clear
C)
\[IgA\]
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 104) Megakaryocytes are the
A)
cells found between the spaces in the lamellae of compact bone tissues
done
clear
B)
cells of bone marrow which give rise to thrombocytes
done
clear
C)
phagocytes found in the lymph
done
clear
D)
another name for thrombocytes.
done
clear
View Answer play_arrow
question_answer 105) The hepatic cells which act as phagocytes are
A)
Hansens cells
done
clear
B)
acinar cells
done
clear
C)
Kupffer cells
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 106) Which of the following is incorrect sequence in classification?
A)
amphibia-anura-frog
done
clear
B)
reptilia-squamata-ophidia-shake
done
clear
C)
aves-neognathae-passeriformes-sparrow
done
clear
D)
mammalia-eutheria-artiodactyla-horse.
done
clear
View Answer play_arrow
question_answer 107) Sea lilies are
A)
echinoderms
done
clear
B)
coelenterates
done
clear
C)
rotifers
done
clear
D)
aquatic plants.
done
clear
View Answer play_arrow
question_answer 108) Enterogastrone secretion is induced by
A)
bile
done
clear
B)
fatty acids.
done
clear
C)
\[HCl\]
done
clear
D)
pancreatic juice
done
clear
View Answer play_arrow
question_answer 109) Binding of ATP to myosin required the cofactor
A)
Mg
done
clear
B)
Na
done
clear
C)
K
done
clear
D)
Ca.
done
clear
View Answer play_arrow
question_answer 110) Cumulus cells are generally found around
A)
corpus luteum
done
clear
B)
ovulated eggs
done
clear
C)
ovary
done
clear
D)
uterus.
done
clear
View Answer play_arrow
question_answer 111) Which of the following chambers of ruminant stomach contains the gastric juice?
A)
abomasum
done
clear
B)
omasum
done
clear
C)
rumen
done
clear
D)
reticulum.
done
clear
View Answer play_arrow
question_answer 112) The embryonic skeleton is formed by
A)
white fibrous cartilage
done
clear
B)
elastic cartilage
done
clear
C)
hyaline cartilage
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 113) Reticular tissue forms the supporting frame work of
A)
bone marrow
done
clear
B)
lymph gland
done
clear
C)
spleen
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 114) Which of the following organisms causes the sexual disease syphilis?
A)
Neisseria gonorrhoae
done
clear
B)
Treponema pallidum
done
clear
C)
Pasteurella pestis
done
clear
D)
Clostrldium botulinum.
done
clear
View Answer play_arrow
question_answer 115) The process by which new alleles of a gene are produced is termed as
A)
point mutation
done
clear
B)
gene manipulation
done
clear
C)
para mutation
done
clear
D)
forward mutation.
done
clear
View Answer play_arrow
question_answer 116) Deposition of uric acid crystals within the synovial joint causes as
A)
osteoarthritis
done
clear
B)
rheumatoid arthritis
done
clear
C)
gout
done
clear
D)
paralysis.
done
clear
View Answer play_arrow
question_answer 117) Aromatic amines used in the manufacture of synthetic dyes cause cancer of the
A)
skin
done
clear
B)
lung
done
clear
C)
liver
done
clear
D)
urinary bladder.
done
clear
View Answer play_arrow
question_answer 118) Allergic reaction can be controlled by
A)
antibiotics
done
clear
B)
histamines
done
clear
C)
antihistamines
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 119) Hepatitis B is transmitted through
A)
blood transfusion
done
clear
B)
intimate physical contact
done
clear
C)
sexually
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 120) The hereditary disease in which the urine of a person turns black on exposure to air due to the presence of homogentisic acid is known as
A)
ketonuria
done
clear
B)
phenylketonuria
done
clear
C)
haematuria
done
clear
D)
alkaptonuria.
done
clear
View Answer play_arrow
question_answer 121) Centre for thermoregulation is located in
A)
anterior pituitary
done
clear
B)
hypothalamus
done
clear
C)
kidney
done
clear
D)
heart.
done
clear
View Answer play_arrow
question_answer 122) In prokaryotes, DNA replication occurs
A)
at the time of cytokinesis
done
clear
B)
soon after cytokinesis
done
clear
C)
well before cytokinesis
done
clear
D)
long after cytokinesis.
done
clear
View Answer play_arrow
question_answer 123) Which of the following is the abundant protein in human body?
A)
haemoglobin
done
clear
B)
myoglobin
done
clear
C)
\[\alpha -\]keratin
done
clear
D)
collagen.
done
clear
View Answer play_arrow
question_answer 124) In zoological nomenclature the sub-species is represented by
A)
trinomen
done
clear
B)
binomen
done
clear
C)
uninomen
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 125) Miller and Ureys experiment in its created atmosphere employed
A)
oxygen, ammonia, methane and water
done
clear
B)
hydrogen, ammonia, methane and water
done
clear
C)
hydrogen, ammonia, ethane and water
done
clear
D)
hydrogen, oxygen, nitrogen and water.
done
clear
View Answer play_arrow
question_answer 126) Most fossils have been found in
A)
black soil
done
clear
B)
lava flows
done
clear
C)
granite
done
clear
D)
sedimentary rocks.
done
clear
View Answer play_arrow
question_answer 127) The Nobel Prize for medicine in 1998 was won by
A)
Ferid Murad
done
clear
B)
Jon Watson
done
clear
C)
V.M. Ingram
done
clear
D)
Southerland.
done
clear
View Answer play_arrow
question_answer 128) Coelomoducts are the excretory organs in
A)
earthworm
done
clear
B)
Hydra
done
clear
C)
Planaria
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 129) Athletes generally suffer from
A)
tachycardia
done
clear
B)
bradycardia
done
clear
C)
dyspnoea
done
clear
D)
cardiac arrythmias.
done
clear
View Answer play_arrow
question_answer 130) Which of the following vitamins serves as a coenzyme in transamination and decarboxylation of amino acid?
A)
pantothenic acid
done
clear
B)
folic acid
done
clear
C)
pyridoxine
done
clear
D)
niacin.
done
clear
View Answer play_arrow
question_answer 131) Cholecystectomy (removal of gall bladder) results in
A)
gastric cramps
done
clear
B)
impaired fat digestion
done
clear
C)
perpetual indigestion
done
clear
D)
no appreciable change.
done
clear
View Answer play_arrow
question_answer 132) The protein most extensively studied in age-related changes is
A)
myosin
done
clear
B)
actin
done
clear
C)
collagen
done
clear
D)
tubulin.
done
clear
View Answer play_arrow
question_answer 133) In saltatory conduction of impulse
A)
time is conserved
done
clear
B)
energy is conserved
done
clear
C)
both time and energy are conserved
done
clear
D)
neither time nor energy is conserved.
done
clear
View Answer play_arrow
question_answer 134) Podocytes are associated with
A)
muscle fibres
done
clear
B)
auditory canal
done
clear
C)
neuron
done
clear
D)
nephron.
done
clear
View Answer play_arrow
question_answer 135) Excretion of potassium is governed primarily by
A)
potassium reabsorption in proximal convoluted tubule
done
clear
B)
potassium secretion in proximal convoluted tubule
done
clear
C)
potassium secretion in distal convoluted tubule
done
clear
D)
potassium reabsorption in distal convoluted tubule.
done
clear
View Answer play_arrow
question_answer 136) Ribosomes are made up of
A)
\[tRNA\] and protein
done
clear
B)
\[rRNA\]and protein
done
clear
C)
\[mRNA\]and protein
done
clear
D)
\[mRNA\] and DNA.
done
clear
View Answer play_arrow
question_answer 137) Duchenne muscular dystrophy, a sex-linked inheritance, is characterized by
A)
sex ratio disorder
done
clear
B)
wasting away of muscles
done
clear
C)
inevitable death
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 138) In transcription, anticodon is seen in
A)
\[tRNA\]
done
clear
B)
\[rRNA\]
done
clear
C)
\[mRNA\]
done
clear
D)
DNA.
done
clear
View Answer play_arrow
question_answer 139) In ECG, P-R interval corresponds to
A)
time delay in\[A-V\]node
done
clear
B)
\[S-A\]nodal conduction time
done
clear
C)
increased ventricular contraction
done
clear
D)
time interval between onset of ventricular contraction.
done
clear
View Answer play_arrow
question_answer 140) During oogenesis the second meiotic division occurs
A)
before ovulation
done
clear
B)
after fertilization
done
clear
C)
just before fertilization
done
clear
D)
immediately after fertilization.
done
clear
View Answer play_arrow
question_answer 141) Human eye is sensitive to wavelength in the range of
A)
300-390 nanometer
done
clear
B)
380-760 nanometer a
done
clear
C)
790-800 nanometer
done
clear
D)
300-360 nanometer.
done
clear
View Answer play_arrow
question_answer 142) Which of the hormones is a polypeptide
A)
LH
done
clear
B)
FSH
done
clear
C)
insulin
done
clear
D)
thyroxine.
done
clear
View Answer play_arrow
question_answer 143) Thyroid hormone has regulatory effect on
A)
protein metabolism
done
clear
B)
carbohydrate metabolism
done
clear
C)
fat metabolism
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 144) Which of the following is glycoprotein?
A)
haemoglobin
done
clear
B)
casein
done
clear
C)
egg albumin
done
clear
D)
gelatin
done
clear
View Answer play_arrow
question_answer 145) A complete catalytically active enzyme together with its coenzyme is termed as
A)
apoenzyme
done
clear
B)
holoenzyme
done
clear
C)
isozyme
done
clear
D)
lysozyme.
done
clear
View Answer play_arrow
question_answer 146) Excessive nutrient load in a water body leads to its
A)
nitrification
done
clear
B)
eutrophication
done
clear
C)
glaciation
done
clear
D)
purification.
done
clear
View Answer play_arrow
question_answer 147) The stable community structure of an ecosystem is termed as
A)
dominant community
done
clear
B)
biocommunity
done
clear
C)
domicile factor
done
clear
D)
climax community.
done
clear
View Answer play_arrow
question_answer 148) The Periyar sanctuary is famous for
A)
elephants
done
clear
B)
wild bears
done
clear
C)
swamp deers
done
clear
D)
antelopes.
done
clear
View Answer play_arrow
question_answer 149) The movement of oxygen from alveoli into the blood in pulmonary capillaries is through
A)
active transport
done
clear
B)
facilitated transport
done
clear
C)
passive transport
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 150) Which of the following are osmoconformers?
A)
bony fishes
done
clear
B)
elasmobranche
done
clear
C)
hagflshes
done
clear
D)
Lampreys.
done
clear
View Answer play_arrow
question_answer 151) Specific proteins responsible for the flow of materials and information into the cell are called
A)
membrane receptors
done
clear
B)
carrier proteins
done
clear
C)
integral proteins
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 152) Proteins that are embedded in the liqid layer of biomembranes are called
A)
intrinsic
done
clear
B)
extrinsic
done
clear
C)
peripheral
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 153) The fluid-mosaic model of biomembrane was proposed by
A)
Waston and Crick
done
clear
B)
Singer and Nicholson
done
clear
C)
Danielli and Davson
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 154) The thickness of cell membranes varies between 6-9
A)
millimeters
done
clear
B)
micrometers
done
clear
C)
nanometers
done
clear
D)
picometers
done
clear
View Answer play_arrow
question_answer 155) The percentage of proteins in the mitochondrial membrane is
A)
20
done
clear
B)
50
done
clear
C)
80
done
clear
D)
100.
done
clear
View Answer play_arrow
question_answer 156) The\[N{{a}^{+}}/{{K}^{+}}\]pump transports
A)
\[3N{{a}^{+}}\]into and\[2{{K}^{+}}\]out of cell
done
clear
B)
\[2N{{a}^{+}}\]into and\[2{{K}^{+}}\]out of cell
done
clear
C)
\[3N{{a}^{+}}\]out of and\[3{{K}^{+}}\]into cell
done
clear
D)
\[3N{{a}^{+}}\] out and\[2{{K}^{+}}\]into cell.
done
clear
View Answer play_arrow
question_answer 157) The bounding layer of protoplasm is called
A)
ectoplasm
done
clear
B)
ectoderm
done
clear
C)
endoplasm
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 158) The maintenance of a steady and stable internal environment in an organism is called
A)
equilibrium
done
clear
B)
adaptation
done
clear
C)
homeostasis
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 159) One of the sciences for which training in biology is essential namely the science of treating diseases with drugs or curative substances, is called
A)
pest control
done
clear
B)
physiotherapy
done
clear
C)
medicine
done
clear
D)
pharmacy.
done
clear
View Answer play_arrow
question_answer 160) Roughly speaking, the ratio of present day species of plants to animals is
A)
\[\begin{matrix} 1.0 & 0.04 \\ \end{matrix}\]
done
clear
B)
\[\begin{matrix} 1.0 & 0.4 \\ \end{matrix}\]
done
clear
C)
\[\begin{matrix} 1.0 & 1.4 \\ \end{matrix}\]
done
clear
D)
\[\begin{matrix} 1.0 & 2.4 \\ \end{matrix}\]
done
clear
View Answer play_arrow
question_answer 161) As they release hydrolases that digest old and damaged cells, the term suicide bags is aptly used by cell biologists for
A)
golgi bodies
done
clear
B)
lysosomes
done
clear
C)
glyoxisomes
done
clear
D)
peroxisomes.
done
clear
View Answer play_arrow
question_answer 162) The conspicuous protein-storing layer of endosperm in cereal grains Contains
A)
ribosomes
done
clear
B)
microbodies
done
clear
C)
aleurone grains
done
clear
D)
starch grains.
done
clear
View Answer play_arrow
question_answer 163) Thread like protoplasmic projections on the free surface of absorptive cells (such as intestinal cells) are called
A)
plasmodesmata
done
clear
B)
microfilaments
done
clear
C)
cilia
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 164) The space separating the two membranes around the nucleus is called
A)
endonuclear space
done
clear
B)
exonuclear space
done
clear
C)
matric space
done
clear
D)
perinuclear space.
done
clear
View Answer play_arrow
question_answer 165) The total number of cells in the human body is at least
A)
\[{{10}^{3}}\]
done
clear
B)
\[{{10}^{6}}\]
done
clear
C)
\[{{10}^{9}}\]
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 166) The catalase-containing vesicles present in liver and leaf cells are called
A)
glyoxisomes
done
clear
B)
lysosomes
done
clear
C)
peroxisomes
done
clear
D)
polyribosomes.
done
clear
View Answer play_arrow
question_answer 167) Dictyosomes were discovered in 1989 by
A)
Robert Brown
done
clear
B)
Rudolf Virchow
done
clear
C)
Robert Hooke
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 168) The two cell organelles not enclosed by a membrane are
A)
chloroplast and mitochondrion
done
clear
B)
nucleus and nucleolus
done
clear
C)
golgi apparatus and microbodies
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 169) The primary cells wall of plant consist of
A)
cellulose
done
clear
B)
hemicellulose
done
clear
C)
pectin
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 170) The layer of cell wall common to two adjacent cell wall in plants is called
A)
middle lamella
done
clear
B)
middle layer
done
clear
C)
common lamella
done
clear
D)
common layer.
done
clear
View Answer play_arrow
question_answer 171) The symbiotic association of an algae and a fungus is called
A)
mycorrhiza
done
clear
B)
fairy ring
done
clear
C)
lichen
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 172) Early and late blight of potato are caused respectively by
A)
Fusarium and Claviceps
done
clear
B)
Gibberella and Penicillium
done
clear
C)
Aspergillus and Ustilago
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 173) Fairy Rings in lawns result from outward- spreading circles of mycelia of mushrooms producing, at their periphery, fruiting bodies called
A)
ascocarps
done
clear
B)
basidiocarps
done
clear
C)
sorocarps
done
clear
D)
pseudocarps.
done
clear
View Answer play_arrow
question_answer 174) The phenomenon of sexual reproduction in zygomycetes, dependent upon the successful mating between\[+\]and\[-\]strains, is called
A)
heterothallism
done
clear
B)
heterosis
done
clear
C)
heterospory
done
clear
D)
heterogamy.
done
clear
View Answer play_arrow
question_answer 175) The number of known species of fungi is about
A)
1,000
done
clear
B)
10,000
done
clear
C)
1,00,000
done
clear
D)
10,00,000.
done
clear
View Answer play_arrow
question_answer 176) The rumen of cattle is the site of fermentation of cellulose fibres through the action of
A)
archaebacteria
done
clear
B)
cyanobacteria
done
clear
C)
mycoplasmas
done
clear
D)
spirochaetes.
done
clear
View Answer play_arrow
question_answer 177) An antibiotic not produced by one of the monera (Streptomyces) is
A)
erythromycin
done
clear
B)
penicillin
done
clear
C)
streptomycin
done
clear
D)
tetracycline
done
clear
View Answer play_arrow
question_answer 178) Gram negative heterotrophic bacteria that can biodegrade a variety of natural as well as man- made organic compounds, such as petroleum products, include
A)
cyanobacteria
done
clear
B)
Clostridia
done
clear
C)
archaebacteria
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 179) Mycoplasmas are also called PPLO, which is an abbreviation of
A)
pentose phosphate-led oxidation
done
clear
B)
parasitic prokaryote-like organisms
done
clear
C)
pleuropneumonia and plant-like organisms
done
clear
D)
pleuropneumonia-like organisms
done
clear
View Answer play_arrow
question_answer 180) The most numerous and ancient, smallest and simplest microscopic organisms belong to which of the following kingdom?
A)
bacteria
done
clear
B)
fungi
done
clear
C)
monera
done
clear
D)
plantae.
done
clear
View Answer play_arrow
question_answer 181) Dodder (Cuscutta sp.) is an exceptional dicotyledon as it
A)
is a parasite
done
clear
B)
is without leaves
done
clear
C)
lacks cotyledons
done
clear
D)
does not produce flowers.
done
clear
View Answer play_arrow
question_answer 182) Chilghoza, a gymnosperm seed, that is eaten as a dry fruit is produced by
A)
Pinus roxburgii
done
clear
B)
Pinus gerardiana
done
clear
C)
Ginkgo biloba
done
clear
D)
Cedrus deofara.
done
clear
View Answer play_arrow
question_answer 183) The seed coat in both gymnosperms and angiosperms is derived from the
A)
megaspore
done
clear
B)
microspore
done
clear
C)
megasporangium
done
clear
D)
microsporangium.
done
clear
View Answer play_arrow
question_answer 184) Conifers have a definite advantage over other plants of the colder regions, due to their sustained photosynthetic activity, being
A)
perennial
done
clear
B)
tall
done
clear
C)
evergreen
done
clear
D)
xerophytic.
done
clear
View Answer play_arrow
question_answer 185) Ciliated reproductive bodies are not found in
A)
Chlamydomonos
done
clear
B)
Spirogyra
done
clear
C)
Ulothrix
done
clear
D)
Volvox.
done
clear
View Answer play_arrow
question_answer 186) The present day higher green plants are believed to have evolved from
A)
ferns
done
clear
B)
green algae
done
clear
C)
liverworts
done
clear
D)
mosses.
done
clear
View Answer play_arrow
question_answer 187) A few exceptional algae are parasitic. They include
A)
Cephaleuros and Harveyella
done
clear
B)
Sargassum and Spirogyra
done
clear
C)
Ulothrix and Volvox
done
clear
D)
Chlamydomonas and Gelidinm.
done
clear
View Answer play_arrow
question_answer 188) The commercially exploited algae include
A)
Caulerpa, Chara and Volvox
done
clear
B)
Gelidium, Laminaria and Porphyra
done
clear
C)
Acetabularia, Spirogyra and Ulothrix
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 189) The red colour of rhodophyta is due to the preponderance of
A)
phycobilins
done
clear
B)
phycocyanin
done
clear
C)
phycoerythrin
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 190) Kingdom plantae comprises multicellular photoautotrophs that are
A)
aquatic
done
clear
B)
epiphytic
done
clear
C)
terrestrial
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 191) The Chipko Movement for conservation of forests was started in 1972 in
A)
North Kanara distric
done
clear
B)
Silent Valley
done
clear
C)
Sunderbans
done
clear
D)
Tehri-Garhwal.
done
clear
View Answer play_arrow
question_answer 192) The government-sponsored programme for growing trees in towns and cities for aesthetic value is called
A)
floriculture
done
clear
B)
agroforestry
done
clear
C)
social forestry
done
clear
D)
urban forestry.
done
clear
View Answer play_arrow
question_answer 193) MAB of UNESCO stands for
A)
man and biodiversity
done
clear
B)
maintenance of acquired biodiversity
done
clear
C)
movement for auxiliary biosphere
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 194) The rate of formation of organic molecules in green plants or their populations is called
A)
photosynthetic rate
done
clear
B)
net assimilation rate
done
clear
C)
gross primary productivity
done
clear
D)
net primary productivity.
done
clear
View Answer play_arrow
question_answer 195) The natural cycles of carbon, oxygen, nitrogen, water, phosphorous, sulphur etc. are collectively called as
A)
biochemical cycles
done
clear
B)
geochemical cycles
done
clear
C)
material cycles
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 196) Recycling of matter occurs between the biotic and abiotic components of the biosphere during the formers
A)
life
done
clear
B)
death
done
clear
C)
both [a] and [b]
done
clear
D)
neither [a] nor [b].
done
clear
View Answer play_arrow
question_answer 197) With regard to materials, the earth and its atmosphere are a closed system. However, a little inflow of material does occur from outer space in the form of
A)
UFOs
done
clear
B)
cosmic rays
done
clear
C)
meteros
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 198) Biosphere may be defined as
A)
earths hydrosphere, lithosphere and atmosphere
done
clear
B)
a combination of all ecosystems of the earths environment
done
clear
C)
the entire inhabited part of the earth and its atmosphere, including living and non-living matter
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 199) Of the known flowering plants, the number of species of monocots is a partly
A)
5000
done
clear
B)
50,000
done
clear
C)
5,00,000
done
clear
D)
555,00,000.
done
clear
View Answer play_arrow
question_answer 200) The gymnosperms formed the dominant vegetation on the earth during the geological period called
A)
carboniferous
done
clear
B)
permian
done
clear
C)
triassic
done
clear
D)
Jurassic.
done
clear
View Answer play_arrow