question_answer 1) A block is resting on a piston which is moving vertically with SHM of period 1 s. At what amplitude of motion will the block and piston separate?
A)
0.2 m
done
clear
B)
0.25 m
done
clear
C)
0.3 m
done
clear
D)
0.35 m
done
clear
View Answer play_arrow
question_answer 2) Two simple harmonic motions are given by \[y=A\sin \left( \omega t+\delta \right)\]and \[y=A\sin \left( \omega t+\delta +\frac{\pi }{2} \right)\] act on a panicle simultaneously, then the motion of particle will be
A)
circular anti-clockwise
done
clear
B)
elliptical anti-clockwise
done
clear
C)
elliptical clockwise
done
clear
D)
circular clockwise
done
clear
View Answer play_arrow
question_answer 3) Two sources are at a finite distance apart. They emit sound of wavelength\[\lambda \]. An observer situated between them on line joining the sources, approaches towards one source with speed u, then the number of beats heard per second by observer will be
A)
\[\frac{2u}{\lambda }\]
done
clear
B)
\[\frac{u}{\lambda }\]
done
clear
C)
\[\frac{u}{2\lambda }\]
done
clear
D)
\[\frac{\lambda }{u}\]
done
clear
View Answer play_arrow
question_answer 4) A man goes at the top of a smooth inclined plane. He releases a bag to fall freely and himself slides down on inclined plane to reach the bottom. If u1, and u2 are the velocities of the man and bag respectively, then
A)
\[{{u}_{1}}>{{u}_{2}}\]
done
clear
B)
\[{{u}_{1}}<{{u}_{2}}\]
done
clear
C)
\[{{u}_{1}}={{u}_{2}}\]
done
clear
D)
\[{{u}_{1}}\,and\,{{u}_{2}}\,cannot\,be\,compared\]
done
clear
View Answer play_arrow
question_answer 5) Two planets A and B have the same material density. If the radius of A is twice that of B, then the ratio of escape velocity \[\frac{{{V}_{A}}}{{{V}_{B}}}\]is
A)
2
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
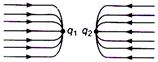
question_answer 6)
The given figure gives electric lines of force due to two charges \[{{q}_{1}}\] and \[{{q}_{2}}\]. What are the signs of the two charges?
A)
Both are negative
done
clear
B)
Both are positive
done
clear
C)
\[{{q}_{1}}\] is positive but \[{{q}_{2}}\] is negative
done
clear
D)
\[{{q}_{1}}\]is negative but \[{{q}_{2}}\] is positive
done
clear
View Answer play_arrow
question_answer 7) Three point charges q, - 2 q and -2 q are placed at the vertices of an equilateral triangle of side a. The work done by some external force to increase their separation to 2a will be
A)
negative
done
clear
B)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{2{{q}^{2}}}{a}\]
done
clear
C)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{3{{q}^{2}}}{a}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 8) A radioactive element x converts into another stable element y. Half-life of x is 2 h, initially only x is present. After time r, the ratio of atoms of x and y is found to be 1 : 4, then t in hour is
A)
2
done
clear
B)
4
done
clear
C)
between 4 and 6
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 9) The shortest wavelength of the Bracken series of hydrogen like atom (atomic number = Z) is the same as the shortest wavelength of the Balmer series of hydrogen atom. The value of Z is
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 10) Radius of gyration of disc of mass 50 g and radius 2.5 cm about an axis passing through its centre of gravity and perpendicular to the plane is
A)
6.54 cm
done
clear
B)
3.64 cm
done
clear
C)
1.77 cm
done
clear
D)
0.88 cm
done
clear
View Answer play_arrow
question_answer 11) Ray optics is valid, when characteristic dimensions are
A)
of the same order as the wavelength of light
done
clear
B)
much smaller than the wavelength of light
done
clear
C)
of the order of one millimeter
done
clear
D)
much larger than the wavelength of light
done
clear
View Answer play_arrow
question_answer 12) A beam of electrons is moving with constant velocity in a region having electric and magnetic fields of strength \[20V{{m}^{-1}}\]and 0.5 T at right angles to the direction of motion of the electrons. What is the velocity of the electrons?
A)
20 \[m{{s}^{-1}}\]
done
clear
B)
40 \[m{{s}^{-1}}\]
done
clear
C)
8 \[m{{s}^{-1}}\]
done
clear
D)
5.5 \[m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 13) Gases begin to conduct electricity at low pressure because
A)
at low pressure, gases turn into plasma
done
clear
B)
colliding electrons can acquire higher kinetic energy due to increased mean free path leading to ionisation of atoms
done
clear
C)
atom breaks up into electrons and protons
done
clear
D)
the electrons in atoms can move freely at low pressure
done
clear
View Answer play_arrow
question_answer 14) A light of wavelength 5890\[\overset{\text{o}}{\mathop{\text{A}}}\,\]falls normally on a thin air film. The minimum thickness of the film such that the film appears dark in reflected light is
A)
\[2.945\times {{10}^{-7}}m\]
done
clear
B)
\[3.945\times {{10}^{-7}}m\]
done
clear
C)
\[4.95\times {{10}^{-7}}m\]
done
clear
D)
\[1.945\times {{10}^{-7}}m\]
done
clear
View Answer play_arrow
question_answer 15) For a black body at temperature \[727{}^\circ C\] its radiating power is 60 W and temperature of surrounding is\[227{}^\circ C\]. If the temperature of the black body is changed to \[1227{}^\circ C,\]then its radiating power will be
A)
120 W
done
clear
B)
240 W
done
clear
C)
304 W
done
clear
D)
320 W
done
clear
View Answer play_arrow
question_answer 16) An object is moving through the liquid. Tit viscous damping force acting on it a proportional to the velocity. Then dimensional formula of constant of proportionality is
A)
\[\left[ M{{L}^{-1}}{{T}^{-1}} \right]\]
done
clear
B)
\[\left[ ML{{T}^{-1}} \right]\]
done
clear
C)
\[\left[ {{M}^{0}}L{{T}^{-1}} \right]\]
done
clear
D)
\[\left[ M{{L}^{0}}{{T}^{-1}} \right]\]
done
clear
View Answer play_arrow
question_answer 17) A car moves on a circular road. It describes equal angles about the centre in equal intervals of time. Which of the following statements about the velocity of the earn true?
A)
Magnitude of velocity is not constant
done
clear
B)
Both magnitude and direction of below change
done
clear
C)
Velocity is directed towards the centered the circle
done
clear
D)
Magnitude of velocity is constant but direction changes
done
clear
View Answer play_arrow
question_answer 18)
A stone is attached to one end of a string and rotated in a vertical circle. If string breaks at the position of maximum tension, it will break at
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 19) A sealed container with negligible coefficient of volumetric expansion contains helium (a monoatomic gas). When it is heated fm 300 K to 600 K, the average KE of helm atoms is
A)
halved
done
clear
B)
unchanged
done
clear
C)
doubled
done
clear
D)
increased by factor \[\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 20) A convex lens of focal length 20 cm placed in contact with a plane mirror acts as a
A)
convex mirror of focal length 10 cm
done
clear
B)
concave mirror of focal length 40 cm
done
clear
C)
concave mirror of focal length 60 cm
done
clear
D)
concave mirror of focal length 10 cm
done
clear
View Answer play_arrow
question_answer 21) A child is swinging a swing. Minimum and maximum heights of swing from earths surface are 0.75 m and 2 m respectively. The maximum velocity of this swing is
A)
5\[m{{s}^{-1}}\]
done
clear
B)
10\[m{{s}^{-1}}\]
done
clear
C)
15\[m{{s}^{-1}}\]
done
clear
D)
17\[m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 22) At ordinary temperature, the molecules of an ideal gas have only translational and rotational kinetic energies. At high tempera-tures they may also have vibrational energy. As a result of this at higher temperatures (\[{{C}_{V}}\]= molar heat capacity at constant volume)
A)
\[{{C}_{V}}=\frac{3}{2}R\] for a monoatomic gas
done
clear
B)
\[{{C}_{V}}>\frac{3}{2}R\]for a monoatomic gas
done
clear
C)
\[{{C}_{V}}<\frac{5}{2}R\]for a diatomic gas
done
clear
D)
\[{{C}_{V}}=\frac{5}{2}R\]for a diatomic gas
done
clear
View Answer play_arrow
question_answer 23) Work done per mol in an isothermal change is
A)
\[RT{{\log }_{10}}\frac{{{V}_{2}}}{{{V}_{1}}}\]
done
clear
B)
\[RT{{\log }_{10}}\frac{{{V}_{1}}}{{{V}_{2}}}\]
done
clear
C)
\[RT{{\log }_{e}}\frac{{{V}_{2}}}{{{V}_{1}}}\]
done
clear
D)
\[RT{{\log }_{e}}\frac{{{V}_{1}}}{{{V}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 24) For a given plate-voltage, the plate current in a triode is maximum when the potential of
A)
the grid is positive and plate is negative
done
clear
B)
the grid is positive and plate is positive
done
clear
C)
the grid is zero and plate is positive
done
clear
D)
the grid is negative and plate is positive
done
clear
View Answer play_arrow
question_answer 25) A wire is wound in the form of a solenoid of length l and distance d. When a strong current is passed through a solenoid, there is a tendency to
A)
increase \[l\] but decrease d
done
clear
B)
keep both \[l\] and d constant
done
clear
C)
decrease \[l\] but increase d
done
clear
D)
increase both \[l\] and d
done
clear
View Answer play_arrow
question_answer 26) The sheave of a galvanometer of resistance 100\[\Omega \] contains 25 divisions. It gives a deflection of one division on passing a current of \[4\times {{10}^{-4}}\text{A}\]. The resistance in ohm to be added to it, so that it may become a voltmeter of range 2.5 V is
A)
150
done
clear
B)
170
done
clear
C)
110
done
clear
D)
220
done
clear
View Answer play_arrow
question_answer 27) A train is moving with a constant speed along a circular track. The engine of the train emits a sound of frequency f. The frequency heard by the guard at rear end of the train is
A)
less than f
done
clear
B)
equal to f
done
clear
C)
is greater than f
done
clear
D)
may be greater than, less than or equal to f depending on the factors like speed of train, length of train and radius of circular track
done
clear
View Answer play_arrow
question_answer 28) If \[{{\lambda }_{1}},{{\lambda }_{2}}\] and \[{{\lambda }_{3}}\]are the wavelengths of the waves giving resonance with the fundamental, first and second overtones respectively of a closed organ pipe. Then the ratio of wavelengths \[{{\lambda }_{1}}:{{\lambda }_{2}}:{{\lambda }_{3}}\]is
A)
\[1:3:5\]
done
clear
B)
\[1:2:3\]
done
clear
C)
\[5:3:1\]
done
clear
D)
\[1:\frac{1}{3}:\frac{1}{5}\]
done
clear
View Answer play_arrow
question_answer 29) By what percent the energy of a satellite has to be increased to shift it from an orbit of radius r to 3/2 r?
A)
15%
done
clear
B)
20.3%
done
clear
C)
66.7%
done
clear
D)
33.33%
done
clear
View Answer play_arrow
question_answer 30) The slope of plate characteristic of a vacuum diode is\[2\times {{10}^{-2}}mA{{V}^{-1}}\]. The plate resistance of diode will be
A)
\[50\Omega \]
done
clear
B)
\[50k\Omega \]
done
clear
C)
\[500\Omega \]
done
clear
D)
\[500k\Omega \]
done
clear
View Answer play_arrow
question_answer 31) A stone is thrown vertically upwards. When stone is at a height half of its maximum height, its speed is 10 \[m{{s}^{-1}},\] then the maximum height attained by the stone is (g = 10 \[m{{s}^{-2}}\])
A)
5 m
done
clear
B)
150 m
done
clear
C)
20 m
done
clear
D)
10 m
done
clear
View Answer play_arrow
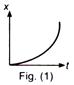
question_answer 32)
Figures (1) and (2) show the displacement-time graphs of two particles moving along the x-axis. We can say that
A)
both the panicles are having a uniform accelerated motion
done
clear
B)
both the particles are having a uniform retarted motion
done
clear
C)
particle (1) is having uniform accelerated motion while particle (2) is having a uniform retarted motion
done
clear
D)
particle (1) is having a uniformly retarted motion while particle (2) is having a uniformly accelerated motion
done
clear
View Answer play_arrow
question_answer 33) A particle of mass m is executing uniform circular motion on a path of radius r. If p is the magnitude of its linear momentum. The radial force acting on the particle is
A)
pmr
done
clear
B)
\[\frac{rm}{p}\]
done
clear
C)
\[\frac{m{{p}^{2}}}{r}\]
done
clear
D)
\[\frac{{{p}^{2}}}{rm}\]
done
clear
View Answer play_arrow
question_answer 34) Water rises to a height h in a capillary tube lowered vertically into a water to a depth l. The lower end of the tube is closed inside the water and the tube is taken out of water and opened. If I < h, then the length of water column remaining in the tube is
A)
zero
done
clear
B)
\[l+h\]
done
clear
C)
\[2\,h\]
done
clear
D)
\[h\]
done
clear
View Answer play_arrow
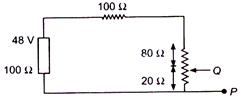
question_answer 35)
In the circuit, the potential difference across PQ will be nearest to
A)
9.6 V
done
clear
B)
6.6 V
done
clear
C)
4.8 V
done
clear
D)
3.2 V
done
clear
View Answer play_arrow
question_answer 36) A ball hits a vertical wall horizontally at 10 \[m{{s}^{-1}}\] and bounces back at 10 \[m{{s}^{-1}}\], then
A)
there is no acceleration because 10ms-1
done
clear
B)
there may be an acceleration because its initial direction is horizontal
done
clear
C)
there is an acceleration because there is a momentum change
done
clear
D)
even though there is no change in momentum there is a change in direction. Hence it has an acceleration
done
clear
View Answer play_arrow
question_answer 37) Sodium and copper have work functions 2.3 eV and 4.5 eV respectively. Then the ratio of their threshold wavelengths is nearest to
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
1 : 4
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 38) Water flows along a horizontal pipe whose cross-section is not constant. The pressure is 1 cm of Hg where the velocity is \[35\text{ }cm{{s}^{-1}}\]a point where the velocity is 65 cms-1, the pressure will be
A)
0.89 cm of Hg
done
clear
B)
8.9 cm of Hg
done
clear
C)
0.5 cm of Hg
done
clear
D)
1 cm of Hg
done
clear
View Answer play_arrow
question_answer 39) In an inductor of self-inductance L = 2 mH, current changes with time according to relation\[I={{t}^{2}}{{e}^{-t}}\]. At what time emf is zero?
A)
3 s
done
clear
B)
4 s
done
clear
C)
1 s
done
clear
D)
2 s
done
clear
View Answer play_arrow
question_answer 40) A capacitor of 10 \[\mu \]F is charged to a potential 50 V with a battery. The battery is n disconnected and an additional day 200\[\mu \]C is given to the positive plate of 1 capacitor. The potential difference across the capacitor will be
A)
100 V
done
clear
B)
60 V
done
clear
C)
80 V
done
clear
D)
50 V
done
clear
View Answer play_arrow
question_answer 41) sA lump of clay of mass 10 g travelling with a velocity of 10\[cm{{s}^{-1}}\]towards east collides head on with another lump of clay of mass 10 g travelling with velocity of 20\[cm{{s}^{-1}}\] towards west. If the two lumps coalesce collision, what is its velocity, if no external force acts on the system?
A)
15 \[cm{{s}^{-1}}\] towards west
done
clear
B)
15 \[cm{{s}^{-1}}\] towards east
done
clear
C)
5 \[cm{{s}^{-1}}\] towards west
done
clear
D)
5 \[cm{{s}^{-1}}\] towards east
done
clear
View Answer play_arrow
question_answer 42) A particle is fired with a speed of \[2\text{ }km{{h}^{-1}}.\] The speed with which it will move in tersteller space is \[({{V}_{e}}=8\sqrt{2}\,km{{h}^{-1}})\]
A)
16.5 \[km{{h}^{-1}}\]
done
clear
B)
11.2 \[km{{h}^{-1}}\]
done
clear
C)
10 \[km{{h}^{-1}}\]
done
clear
D)
8.8 \[km{{h}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 43) A car of mass 1500 kg is moving with a speed of 12.5 \[m{{s}^{-1}}\]on a circular path of radius 20 m on a level road. The value of coefficient friction between the tyres and road, so that the car does not slip, is
A)
0.8
done
clear
B)
0.6
done
clear
C)
0.4
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 44) If \[{{\overrightarrow{a}}_{1}}\] and \[{{\overrightarrow{a}}_{2}}\]are two non-collinear unit vectors and if \[\left| {{\overrightarrow{a}}_{1}}-{{\overrightarrow{a}}_{2}} \right|=\sqrt{3},\]then the value of is \[\left( {{\overrightarrow{a}}_{1}}-{{\overrightarrow{a}}_{2}} \right).\]\[\left( 2{{\overrightarrow{a}}_{1}}+{{\overrightarrow{a}}_{2}} \right)\] is
A)
2
done
clear
B)
3/2
done
clear
C)
1/2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 45) The energy of photon of light is 3 eV. Then the wavelength of photon must be
A)
4125 nm
done
clear
B)
412.5 nm
done
clear
C)
41.250 nm
done
clear
D)
4 nm
done
clear
View Answer play_arrow
question_answer 46) The half-life of radioactive material is 3 h. If the initial amount is 300 g. Then after 18 h, it will remain
A)
4.69 g
done
clear
B)
46.8 g
done
clear
C)
9.375 g
done
clear
D)
93.75 g
done
clear
View Answer play_arrow
question_answer 47) The wavelength of \[{{K}_{a}}\]X-rays for lead isotopes \[P{{b}^{208}},\text{ }P{{b}^{206}}\] and \[P{{b}^{204}}\] are \[{{\lambda }_{1}},{{\lambda }_{2}}\] and \[{{\lambda }_{3}}\]respectively. Then
A)
\[{{\lambda }_{1}}={{\lambda }_{2}}={{\lambda }_{3}}\]
done
clear
B)
\[{{\lambda }_{1}}>{{\lambda }_{2}}>{{\lambda }_{3}}\]
done
clear
C)
\[{{\lambda }_{1}}<{{\lambda }_{2}}<{{\lambda }_{3}}\]
done
clear
D)
\[{{\lambda }_{1}}={{\lambda }_{2}}>{{\lambda }_{3}}\]
done
clear
View Answer play_arrow
question_answer 48) A wire 50 cm long and 1 \[\text{m}{{\text{m}}^{\text{2}}}\]in cross-section carries a current of 4 A when connected to a 2 V battery. The resistivity of the wire is
A)
\[2\times {{10}^{-7}}\Omega \,\,m\]
done
clear
B)
\[5\times {{10}^{-7}}\Omega \,\,m\]
done
clear
C)
\[4\times {{10}^{-6}}\Omega \,\,m\]
done
clear
D)
\[1\times {{10}^{-7}}\Omega \,\,m\]
done
clear
View Answer play_arrow
question_answer 49) A magnet 10 cm long and having a pole strength 2 Am is deflected through \[30{}^\circ \]from the magnetic meridian. The horizontal component of earths induction is \[0.32\times {{10}^{-4}}T,\] then the value of deflecting couple is
A)
\[16\times {{10}^{-7}}Nm\]
done
clear
B)
\[64\times {{10}^{-7}}Nm\]
done
clear
C)
\[48\times {{10}^{-7}}Nm\]
done
clear
D)
\[32\times {{10}^{-7}}Nm\]
done
clear
View Answer play_arrow
question_answer 50) A closely wound flat circular coil of 25 turns of wire has diameter of 10 cm which carries current of 4 A, the flux density at the centre of a coil will be
A)
\[2.28\times {{10}^{-6}}T\]
done
clear
B)
\[1.679\times {{10}^{-6}}T\]
done
clear
C)
\[1.256\times {{10}^{-3}}T\]
done
clear
D)
\[1.572\times {{10}^{-5}}T\]
done
clear
View Answer play_arrow
question_answer 51) A particle of mass 100 g is thrown vertically upwards with a speed of 5\[m{{s}^{-1}}\]. The work done by the force of gravity during the time the particle goes up is
A)
\[-\,0.5\text{ }J\]
done
clear
B)
\[-1.25\text{ }J\]
done
clear
C)
\[1.25\text{ }J\]
done
clear
D)
\[0.5\text{ }J\]
done
clear
View Answer play_arrow
question_answer 52) A mass of M kg is suspended by a weightless string. The horizontal force that is required to displace it until the string makes an angle of \[45{}^\circ \] with the initial vertical direction is
A)
\[Mg\left( \sqrt{2}+1 \right)\]
done
clear
B)
\[Mg\sqrt{2}\]
done
clear
C)
\[\frac{Mg}{\sqrt{2}}\]
done
clear
D)
\[Mg\left( \sqrt{2}-1 \right)\]
done
clear
View Answer play_arrow
question_answer 53)
A force of \[-F\]\[\widehat{k}\]acts on O, the origin of the coordinate system. The torque about the point (1,-1) is
A)
\[F\left( \widehat{i}-\widehat{j} \right)\]
done
clear
B)
\[-F\left( \widehat{i}+\widehat{j} \right)\]
done
clear
C)
\[F\left( \widehat{i}+\widehat{j} \right)\]
done
clear
D)
\[-F\left( \widehat{i}-\widehat{j} \right)\]
done
clear
View Answer play_arrow
question_answer 54) If \[{{M}_{O}}\] is the mass of an oxygen isotope \[_{8}{{O}^{17}},\] \[{{M}_{p}}\]and \[{{M}_{n}}\]are the masses of a proton and a neutron, respectively, the nuclear binding energy of the isotope is
A)
\[\left( {{M}_{O}}-8{{M}_{p}} \right){{c}^{2}}\]
done
clear
B)
\[\left( {{M}_{O}}-8{{M}_{p}}-9{{M}_{n}} \right){{c}^{2}}\]
done
clear
C)
\[{{M}_{O}}{{c}^{2}}\]
done
clear
D)
\[\left( {{M}_{O}}-17{{M}_{n}} \right){{c}^{2}}\]
done
clear
View Answer play_arrow
question_answer 55) A sound absorber attenuates the sound level by 20 dB. The intensity decreases by a factor of
A)
1000
done
clear
B)
10000
done
clear
C)
10
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 56) Which of the following parameters does not characterize the thermodynamic state of matter?
A)
Temperature
done
clear
B)
Pressure
done
clear
C)
Work
done
clear
D)
Volume
done
clear
View Answer play_arrow
question_answer 57) A charged oil drop is suspended in uniform field of \[3\times {{10}^{4}}V{{m}^{-1}},\]so that it neither falls nor rises. The charge on the drop will be (Take the mass of the charge \[=9.9\times {{10}^{-15}}\]kg and g = 10\[m{{s}^{-2}}\])
A)
\[3.3\times {{10}^{-18}}C\]
done
clear
B)
\[3.2\times {{10}^{-18}}C\]
done
clear
C)
\[1.6\times {{10}^{-18}}C\]
done
clear
D)
\[4.8\times {{10}^{-18}}C\]
done
clear
View Answer play_arrow
question_answer 58) A vertical spring with force constant k is fixed on a table. A ball of mass m at a height h above the free upper end of the spring falls vertically on the spring, so that the spring is compressed by a distance d. The net work done in the process is
A)
\[mg\left( h+d \right)+\frac{1}{2}k{{d}^{2}}\]
done
clear
B)
\[mg\left( h+d \right)-\frac{1}{2}k{{d}^{2}}\]
done
clear
C)
\[mg\left( h-d \right)-\frac{1}{2}k{{d}^{2}}\]
done
clear
D)
\[mg\left( h-d \right)+\frac{1}{2}k{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) Dimensions of resistance in an electrical circuit, in terms of dimension of mass M, of length L, of time T and of current I, would be
A)
\[\left[ M{{L}^{2}}{{T}^{3}}{{T}^{-1}} \right]\]
done
clear
B)
\[\left[ M{{L}^{2}}{{T}^{-2}} \right]\]
done
clear
C)
\[\left[ M{{L}^{2}}{{T}^{-1}}{{I}^{-1}} \right]\]
done
clear
D)
\[\left[ M{{L}^{2}}{{T}^{-3}}{{I}^{-2}} \right]\]
done
clear
View Answer play_arrow
question_answer 60) An alpha nucleus of energy \[\frac{1}{2}m{{v}^{2}}\]bombards a heavy nuclear target of charged Ze. Then the distance of closest approach for the alpha nucleus will be proportional to
A)
\[{{v}^{2}}\]
done
clear
B)
\[1/m\]
done
clear
C)
\[1\text{/}{{v}^{4}}\]
done
clear
D)
\[1/Ze\]
done
clear
View Answer play_arrow
question_answer 61) Benzene reacts with\[C{{H}_{3}}COCl\]in the presence of \[AlC{{l}_{3}}\]and gives
A)
\[{{C}_{6}}{{H}_{5}}COC{{H}_{3}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
D)
\[{{C}_{7}}{{H}_{14}}\]
done
clear
View Answer play_arrow
question_answer 62) Lucas test is given fastly by
A)
butanol-2
done
clear
B)
butanol-1
done
clear
C)
iso-butanol
done
clear
D)
2-methyl-2-propanol
done
clear
View Answer play_arrow
question_answer 63) \[N{{H}_{3}}\] gas is passed over heated copper oxide. It oxidises to
A)
\[{{N}_{2}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[HN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Which of the following compounds shows optical isomerism?
A)
\[C{{H}_{3}}-CH-BrCOOH\]
done
clear
B)
\[C{{H}_{2}}OHC{{H}_{2}}-COOH\]
done
clear
C)
\[COOHCHBr-COOH\]
done
clear
D)
\[C{{H}_{2}}Br-C{{H}_{2}}-COOH\]
done
clear
View Answer play_arrow
question_answer 65) \[C{{H}_{2}}=C{{H}_{2}}\xrightarrow{HBr}X\xrightarrow{Aq.\,KOH}Y\xrightarrow[{{I}_{2}}]{N{{a}_{2}}C{{O}_{3}}}Z\] In the above reaction sequence, Z is
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}I\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[CH{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 66) \[{{C}_{6}}{{H}_{5}}Cl+C{{H}_{3}}Cl\xrightarrow{Na/dry\,ether}{{C}_{6}}{{H}_{5}}C{{H}_{3}}+2NaCl\] This reaction is an example of
A)
Wurtz reaction
done
clear
B)
Fittig reaction
done
clear
C)
Wurtz-Fittig reaction
done
clear
D)
Frankland reaction
done
clear
View Answer play_arrow
question_answer 67) The main difference between formic acid and acetic acid is that formic acid is
A)
less acidic
done
clear
B)
dehydrating agent
done
clear
C)
reducing agent
done
clear
D)
bleaching agent
done
clear
View Answer play_arrow
question_answer 68) Hinsberg reagent, \[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\]does not react with
A)
\[1{}^\circ \]amine
done
clear
B)
\[2{}^\circ \]amine
done
clear
C)
\[3{}^\circ \]amine
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 69) Ordinary glass is formed by mixing of
A)
\[N{{a}_{2}}C{{O}_{3}}+CaC{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}+CaC{{O}_{3}}\]+silica
done
clear
C)
silica + \[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
silica + borax
done
clear
View Answer play_arrow
question_answer 70) Correct order of freezing point of 1 M solution of sucrose, \[KCl,\]\[BaC{{l}_{2}}\] and \[AlC{{l}_{3}}\]is
A)
Sucrose \[>KCl>BaC{{l}_{2}}>AlC{{l}_{3}}\]
done
clear
B)
\[AlC{{l}_{3}}>BaC{{l}_{2}}>KCl>\] Sucrose
done
clear
C)
\[BaC{{l}_{2}}>KCl>AlC{{l}_{3}}>\] Sucrose
done
clear
D)
\[KCl>BaC{{l}_{2}}>AlC{{l}_{3}}>\]Sucrose
done
clear
View Answer play_arrow
question_answer 71) Table sugar is
A)
sucrose
done
clear
B)
glucose
done
clear
C)
fructose
done
clear
D)
maltose
done
clear
View Answer play_arrow
question_answer 72) If \[\frac{3}{4}\] quantity of a radioactive substance disintegrates in 60 min, its half-life period will be
A)
15 min
done
clear
B)
half an hour
done
clear
C)
one hour
done
clear
D)
one day
done
clear
View Answer play_arrow
question_answer 73) Element with atomic number 81, is present in which block?
A)
s-block
done
clear
B)
p-block
done
clear
C)
d-block
done
clear
D)
f-block
done
clear
View Answer play_arrow
question_answer 74) Correct order of basic strength is
A)
\[Mg{{(OH)}_{2}}>NaOH>Al{{(OH)}_{3}}\]
done
clear
B)
\[Mg{{(OH)}_{2}}>Al{{(OH)}_{3}}>NaOH\]
done
clear
C)
\[NaOH>Mg{{(OH)}_{2}}>Al{{(OH)}_{3}}\]
done
clear
D)
\[Al{{(OH)}_{3}}>Mg{{(OH)}_{2}}>NaOH\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following will give carbylamines test?
A)
N, N-dimethyl aniline
done
clear
B)
2, 4-dimethyl aniline
done
clear
C)
N-methyl-2-methylaniline
done
clear
D)
N-methyl benzylamine
done
clear
View Answer play_arrow
question_answer 76) Consider following reactions \[I:C(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}CO(g);\,\,\Delta {{H}_{1}}=a\] \[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g);\,\,\Delta {{H}_{2}}=b\] \[C(s)+C{{O}_{2}}(g)\xrightarrow{{}}2C{{O}_{2}}(g);\,\,\Delta {{H}_{3}}=c\]Select correct statement
A)
Heat of formation of \[C{{O}_{2}}\]is (a + b)
done
clear
B)
Heat of combustion of\[C\]is (a + b)
done
clear
C)
\[\Delta {{H}_{3}}=\Delta {{H}_{1}}-\Delta {{H}_{2}}\]
done
clear
D)
All the above are correct statements
done
clear
View Answer play_arrow
question_answer 77) Following reaction is catalysed by \[B{{r}^{-}}\](aq). \[2{{H}_{2}}{{O}_{2}}(aq)\xrightarrow{{}}2{{H}_{2}}O(l)+{{O}_{2}}(g)\] This is an example of
A)
homogeneous catalysis
done
clear
B)
heterogeneous catalysis
done
clear
C)
autocatalysis
done
clear
D)
enzyme catalysis
done
clear
View Answer play_arrow
question_answer 78) Ethylidene dibromide \[\xrightarrow{A}CH\equiv CH\]A is
A)
aq. KOH
done
clear
B)
ale. KOH
done
clear
C)
cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 79) \[{{I}_{2}}+2{{S}_{2}}O_{3}^{2-}\xrightarrow{{}}{{S}_{4}}O_{6}^{2-}+2{{I}^{-}}\] In the above reaction
A)
iodine is reduced; sulphur is reduced
done
clear
B)
iodine is reduced; sulphur is oxidized
done
clear
C)
iodine is oxidised; sulphur is reduced
done
clear
D)
iodine is oxidised; sulphur is oxidized
done
clear
View Answer play_arrow
question_answer 80) Which is the strongest acid?
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[CH\equiv CH\]
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 81) Number of spectral lines of Lyman series of electron when it jumps from 6 to first level (in Lyman series), is
A)
9
done
clear
B)
12
done
clear
C)
15
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 82) Which of the following compounds does liberate \[C{{O}_{2}}\] from\[NaHC{{O}_{3}}\]?
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{4}}{{N}^{+}}O{{H}^{-}}\]
done
clear
D)
\[C{{H}_{3}}\overset{+}{\mathop{N}}\,{{H}_{3}}C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 83) \[\begin{align} & {{H}_{3}}C-CH=C-C{{H}_{2}}-C{{H}_{3}} \\ & \underset{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}}{\mathop{|}}\, \\ \end{align}\]Correct IUPAC name is
A)
3-ethylhex-2-ene
done
clear
B)
3-ethylpent-2-ene
done
clear
C)
3-ethylpent-3-ene
done
clear
D)
3-propylpent-2-ene
done
clear
View Answer play_arrow
question_answer 84) In an organic compound, C = 68.5% and H = 4.91% . Which empirical formula is correct for it?
A)
\[{{C}_{6}}{{H}_{10}}\]
done
clear
B)
\[{{C}_{7}}{{H}_{6}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{5}}{{H}_{8}}O\]
done
clear
D)
\[{{C}_{9}}{{H}_{3}}O\]
done
clear
View Answer play_arrow
question_answer 85) \[E{}^\circ \]for \[M{{g}^{2+}}\text{/}Mg=-\,2.37V,\]\[Z{{n}^{+2}}/Zn\]\[=-\,0.76\,V\] and \[F{{e}^{2+}}/Fe=-\,0.44\,V.\]Which statement is correct?
A)
Zn reduces \[F{{e}^{2+}}\]
done
clear
B)
Zn reduces \[M{{g}^{2+}}\]
done
clear
C)
Mg oxidises \[Fe\]
done
clear
D)
Zn oxidises\[Fe\]
done
clear
View Answer play_arrow
question_answer 86) Enolic form of acetone has
A)
\[8\sigma ,\]\[1\,\pi \]; 2 lone pairs
done
clear
B)
\[9\sigma ,\]\[2\,\pi \]; lone pair
done
clear
C)
\[8\sigma ,\]\[2\,\pi \]; lone pair
done
clear
D)
\[9\sigma ,\]\[1\,\pi \]; 2 lone pairs
done
clear
View Answer play_arrow
question_answer 87) 0.126 g of an acid is titrated with 0.1 N 20 mL of an base. The equivalent weight of the acid is
A)
63
done
clear
B)
50
done
clear
C)
53
done
clear
D)
23
done
clear
View Answer play_arrow
question_answer 88) Darking of surfaces painted with white lead is due to
A)
\[{{H}_{2}}S\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[Cu\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 89) Which of the following will give \[{{H}_{2}}\] gas with dilute\[HN{{O}_{3}}\]?
A)
Mg
done
clear
B)
Zn
done
clear
C)
Cu
done
clear
D)
Hg
done
clear
View Answer play_arrow
question_answer 90) When alkyl aryl ether is heated with HI, it gives
A)
alcohol and phenol
done
clear
B)
alcohol and aryl halide
done
clear
C)
phenol and alkyl halide
done
clear
D)
alkyl halide and aryl halide
done
clear
View Answer play_arrow
question_answer 91) Which of the following is the purest commercial form of iron?
A)
Cast iron
done
clear
B)
Steel
done
clear
C)
Wrought iron
done
clear
D)
Pig iron
done
clear
View Answer play_arrow
question_answer 92) What is the oxidation number of Fe in\[Fe{{(CO)}_{5}}\]?
A)
+ 3
done
clear
B)
zero
done
clear
C)
+ 2
done
clear
D)
+ 5
done
clear
View Answer play_arrow
question_answer 93) Fog is a colloidal solution of
A)
gaseous particles dispersed in liquid
done
clear
B)
liquid dispersed in gas
done
clear
C)
gaseous particles dispersed in gas
done
clear
D)
solid dispersed in gas
done
clear
View Answer play_arrow
question_answer 94) A petrol pump hose pipe for delivery of petrol is made up of
A)
natural rubber
done
clear
B)
vulcanised rubber
done
clear
C)
neoprene
done
clear
D)
butadiene rubber
done
clear
View Answer play_arrow
question_answer 95) German silver is an alloy of
A)
Cu and Zn
done
clear
B)
Cu and Ag
done
clear
C)
Cu and Sn
done
clear
D)
Cu, Zn and Ni
done
clear
View Answer play_arrow
question_answer 96) On increasing pressure, melting point of ice
A)
decreases
done
clear
B)
increases
done
clear
C)
remains unchanged
done
clear
D)
changes in regular manner
done
clear
View Answer play_arrow
question_answer 97) Identify Z in the following sequence of reactions. \[C{{H}_{3}}COOH\xrightarrow{N{{H}_{3}}}X\xrightarrow{\Delta }Y\xrightarrow{{{P}_{2}}{{O}_{5}}}Z\]
A)
\[C{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}CN\]
done
clear
D)
\[C{{H}_{3}}CO{{O}^{-}}NH_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following is the most stable carbocation?
A)
\[{{C}_{6}}{{H}_{5}}CH_{2}^{+}\]
done
clear
B)
\[C{{H}_{3}}CH_{2}^{+}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{C}}\,H\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}{{C}^{+}}\]
done
clear
View Answer play_arrow
question_answer 99) Dehydration of methyl alcohol with concentrated \[{{H}_{2}}S{{O}_{4}}\]yields
A)
methane
done
clear
B)
ethane
done
clear
C)
dimethyl ether
done
clear
D)
acetone
done
clear
View Answer play_arrow
question_answer 100) 60 ml of \[\frac{N}{5}{{H}_{2}}S{{O}_{4}},\]10 mL of \[\frac{N}{5}HN{{O}_{3}}\] and 30 mL of\[\frac{N}{10}HCl\]are mixed together. The strength of the resulting mixture is
A)
0.1 N
done
clear
B)
0.2 N
done
clear
C)
0.3 N
done
clear
D)
0.4 N
done
clear
View Answer play_arrow
question_answer 101) A gas can be liquefied
A)
at its critical temperature
done
clear
B)
above its critical temperature
done
clear
C)
below its critical temperature
done
clear
D)
at 0°C
done
clear
View Answer play_arrow
question_answer 102) The compound that is considered as a true peroxide, is
A)
\[Pb{{O}_{2}}\]
done
clear
B)
\[Ba{{O}_{2}}\]
done
clear
C)
\[Mn{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following oxides of nitrogen does react with \[FeS{{O}_{4}}\] to form a brown compound in the test of nitrate?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[NO\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 104) For the equilibrium,\[2N{{O}_{2}}(g)\rightleftharpoons {{N}_{2}}{{O}_{4}}(g)+14.6\,\,\text{kcal,}\]increase in temperature
A)
favours the formation of\[{{N}_{2}}{{O}_{4}}\]
done
clear
B)
favours the decomposition of\[{{N}_{2}}{{O}_{4}}\]
done
clear
C)
does not affect equilibrium
done
clear
D)
stop the reaction
done
clear
View Answer play_arrow
question_answer 105) A certain mass of the oxygen gas occupies 7 L volume under a pressure of 380 mm Hg. The volume of the same mass of the gas at standard pressure, with temperature remaining constant, shall be
A)
26.60 L
done
clear
B)
54.28 L
done
clear
C)
3.5 L
done
clear
D)
7 L
done
clear
View Answer play_arrow
question_answer 106) Peroxide bond is absent in
A)
\[{{({{S}_{2}}{{O}_{7}})}^{2-}}\]
done
clear
B)
\[{{({{S}_{2}}{{O}_{8}})}^{2-}}\]
done
clear
C)
\[Cr{{O}_{5}}\]
done
clear
D)
\[Ba{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 107) Two acids A and B have \[p{{K}_{a}}\] 4 and 6, then
A)
A is 4/6 times stronger than B
done
clear
B)
A is 10 times stronger than B
done
clear
C)
A is 6/4 times stronger than B
done
clear
D)
B is 10 times stronger than A
done
clear
View Answer play_arrow
question_answer 108) Which compound is expected to be coloured?
A)
\[CuCl\]
done
clear
B)
\[Cu{{F}_{2}}\]
done
clear
C)
\[A{{g}_{2}}S{{O}_{4}}\]
done
clear
D)
\[Mg{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 109) Which compound is insoluble in dilute \[HN{{O}_{3}}\] and dissolved in aqua regia?
A)
\[HgS\]
done
clear
B)
\[CuS\]
done
clear
C)
\[B{{i}_{2}}{{S}_{3}}\]
done
clear
D)
\[PbS\]
done
clear
View Answer play_arrow
question_answer 110) The process of converting hydrated alumina into anhydrous alumina is called
A)
roasting
done
clear
B)
smelting
done
clear
C)
dressing
done
clear
D)
calcination
done
clear
View Answer play_arrow
question_answer 111) Oxidation state of oxygen in \[{{F}_{2}}O\]is
A)
+ 1
done
clear
B)
- 1
done
clear
C)
+ 2
done
clear
D)
- 2
done
clear
View Answer play_arrow
question_answer 112) In III group precipitation, \[N{{H}_{4}}Cl\]is added before adding \[N{{H}_{4}}OH\]to
A)
decrease cone. of \[O{{H}^{-}}\]
done
clear
B)
prevent interference of \[PO_{4}^{3-}\]
done
clear
C)
increase cone. of \[C{{l}^{-}}\]
done
clear
D)
increase cone. of \[O{{H}^{-}}\]ion
done
clear
View Answer play_arrow
question_answer 113) Steel is heated to below red heat and then, cooled slowly. The process refers to
A)
hardening
done
clear
B)
annealing
done
clear
C)
tempering
done
clear
D)
nitriding
done
clear
View Answer play_arrow
question_answer 114) Which one of the following reactions, is called Rosenmund reaction?
A)
Aldehydes are reduced to alcohols
done
clear
B)
Acids are converted to acid chlorides
done
clear
C)
Alcohols are .reduced to hydrocarbons
done
clear
D)
Acid chlorides are reduced to aldehydes
done
clear
View Answer play_arrow
question_answer 115) IUPAC name of\[C{{H}_{3}}-C{{H}_{2}}-\underset{C{{H}_{3}}}{\mathop{\underset{\mathbf{|}}{\mathop{\underset{C{{H}_{2}}}{\mathop{\underset{\mathbf{|}}{\mathop{CH}}\,}}\,}}\,}}\,-\underset{C{{H}_{3}}}{\mathop{\underset{\mathbf{|}}{\mathop{C}}\,}}\,=C{{H}_{2}}\]
A)
2-methyl-3-ethyl-1-pentene
done
clear
B)
3-ethyl-4-methyl-4-pentene
done
clear
C)
3-ethyl-2-methyl-1-pentene
done
clear
D)
3-methyl-2-ethyl-1-pentene
done
clear
View Answer play_arrow
question_answer 116) Which of the following dissolves in water but does not give any oxyacid solution?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[O{{F}_{2}}\]
done
clear
C)
\[SC{{l}_{4}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) The formula of Calgon, used for water softening is
A)
\[N{{a}_{2}}[N{{a}_{4}}{{(P{{O}_{3}})}_{6}}]\]
done
clear
B)
\[N{{a}_{4}}[N{{a}_{2}}{{(P{{O}_{3}})}_{6}}]\]
done
clear
C)
\[N{{a}_{2}}[N{{a}_{4}}{{(P{{O}_{4}})}_{5}}]\]
done
clear
D)
\[N{{a}_{4}}[N{{a}_{4}}{{(P{{O}_{4}})}_{6}}]\]
done
clear
View Answer play_arrow
question_answer 118) A mixture of amylose and amylopectin is called
A)
lactose
done
clear
B)
starch
done
clear
C)
cellulose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 119) Methyl alcohol when reacted with carbon monoxide using cobalt or rhodium as catalyst, compound A is formed. On heating A with HI in the presence of red phosphorus as catalyst B is formed. Identify B.
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}\cdot CHO\]
done
clear
C)
\[C{{H}_{3}}\cdot C{{H}_{2}}\cdot I\]
done
clear
D)
\[C{{H}_{3}}\cdot C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 120) One gas bleaches the colour of flowers by reduction and another gas by oxidation. The gases respectively are
A)
\[S{{O}_{2}}\]and \[C{{l}_{2}}\]
done
clear
B)
\[CO\]and \[C{{l}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\]and\[S{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}S\]and \[B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 121) The term totipotency refers to
A)
the capability of organism to regenerate its lost parts
done
clear
B)
capability of somatic cells to produce complete organism
done
clear
C)
the introduction of foreign gene in a cells DNA
done
clear
D)
the technique of growing immature embryos
done
clear
View Answer play_arrow
question_answer 122) The deteriorative processes in plants, that naturally terminate their functional life, are collectively called
A)
wilting
done
clear
B)
abscission
done
clear
C)
plasmolysis
done
clear
D)
senescence
done
clear
View Answer play_arrow
question_answer 123) Which pigment involves in photoperiodic change in plants?
A)
Phytochrome
done
clear
B)
Cytochrome
done
clear
C)
Chlorophyll
done
clear
D)
Anthocyanin
done
clear
View Answer play_arrow
question_answer 124) Linnaean system of plant classification is based on
A)
morphological and anatomical characters
done
clear
B)
evolutionary trends
done
clear
C)
floral characters
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 125) Succession on secondary base area is
A)
primosere
done
clear
B)
sub sere
done
clear
C)
xerosere
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 126) An unrestricted reproductive capacity is called
A)
birth rate
done
clear
B)
biotic potential
done
clear
C)
carrying capacity
done
clear
D)
fertility
done
clear
View Answer play_arrow
question_answer 127) Alginic acid is found in the cell wall of
A)
Gigartina
done
clear
B)
Laminaria
done
clear
C)
Gelidium
done
clear
D)
Scytonema
done
clear
View Answer play_arrow
question_answer 128) Lady finger (bhindi) belongs to
A)
Malvaceae
done
clear
B)
Cruciferae
done
clear
C)
Solanaceae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 129) Ginger multiplies vegetative by
A)
bud
done
clear
B)
tuber
done
clear
C)
stem
done
clear
D)
rhizome
done
clear
View Answer play_arrow
question_answer 130) In Cycas stem, open vascular bundle is characterized by
A)
phloem being sand witched between xylem
done
clear
B)
cambium present in between xylem and phloem
done
clear
C)
xylem being sand witched between phloem
done
clear
D)
xylem and phloem occurring on different radii
done
clear
View Answer play_arrow
question_answer 131) From which part of coconut coir is obtained?
A)
Pericarp
done
clear
B)
Mesocarp
done
clear
C)
Epicarp
done
clear
D)
Endocarp
done
clear
View Answer play_arrow
question_answer 132) In Funaria, the stomata are found on
A)
foot
done
clear
B)
seta
done
clear
C)
capsule
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133) Tracheophyta consists of
A)
bryophytes only
done
clear
B)
pteridophytes only
done
clear
C)
gymnosperms and angiosperms
done
clear
D)
Both (a) and (c)
done
clear
View Answer play_arrow
question_answer 134) Green-house effect is mainly caused by
A)
CFCs
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
CO
done
clear
View Answer play_arrow
question_answer 135) Male gametophyte of angiosperms is reduced to
A)
one cell
done
clear
B)
two cells
done
clear
C)
three cells
done
clear
D)
four cells
done
clear
View Answer play_arrow
question_answer 136) In\[{{C}_{3}}\]plants, the first stable product of photosynthesis during dark reaction is
A)
PGAL
done
clear
B)
RuBP
done
clear
C)
PGA
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 137) The first \[C{{O}_{2}}\]acceptor in \[{{C}_{4}}\]cycle is
A)
RuBP
done
clear
B)
PEP
done
clear
C)
PGA
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 138) The water available to plants for absorption
A)
gravitational water
done
clear
B)
hygroscopic water
done
clear
C)
capillary water
done
clear
D)
chemically bound water
done
clear
View Answer play_arrow
question_answer 139) Cell wall of fungi is made up of
A)
fungal cellulose
done
clear
B)
hemicellulose
done
clear
C)
fungal chitin
done
clear
D)
Both (a) and (c)
done
clear
View Answer play_arrow
question_answer 140) During cell cycle, RNA and non- histone proteins are synthesized in
A)
S- phase
done
clear
B)
\[{{\text{G}}_{\text{0}}}\text{-}\] phase
done
clear
C)
\[{{\text{G}}_{2}}\text{-}\] phase
done
clear
D)
M- phase
done
clear
View Answer play_arrow
question_answer 141) Bract is a modified
A)
petal
done
clear
B)
sepal
done
clear
C)
leaf
done
clear
D)
involucres
done
clear
View Answer play_arrow
question_answer 142) Hormone replacing the requirement vernalization is
A)
ethylene
done
clear
B)
auxin
done
clear
C)
gibberellins
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 143) Thigmotropism is best seen in
A)
tendrils
done
clear
B)
leaf apex
done
clear
C)
root apex
done
clear
D)
stem apex
done
clear
View Answer play_arrow
question_answer 144) Transpiration is measured by
A)
potometrer
done
clear
B)
porometrer
done
clear
C)
auxanometrer
done
clear
D)
respirometrer
done
clear
View Answer play_arrow
question_answer 145) The most common type of ovule in angiosperms is
A)
amphitropous
done
clear
B)
atropous
done
clear
C)
anatropous
done
clear
D)
circinotropous
done
clear
View Answer play_arrow
question_answer 146) When two hybrids rrTt and Rrtt are crossed, the phenotype ratio of offspring would be
A)
3 : 1
done
clear
B)
9 : 3 : 3 : 1
done
clear
C)
1 : 1
done
clear
D)
1 : 1 : 1 : 1
done
clear
View Answer play_arrow
question_answer 147) One of the most resistant known biological material is
A)
lignin
done
clear
B)
hemicellulose
done
clear
C)
sporopollenin
done
clear
D)
lignocelluloses
done
clear
View Answer play_arrow
question_answer 148) Energy enters the ecosystem through
A)
herbivore
done
clear
B)
carnivore
done
clear
C)
producer
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 149) In soil profile, humus is present in
A)
horizon- O
done
clear
B)
horizon- A
done
clear
C)
horizon- B
done
clear
D)
horizon- C
done
clear
View Answer play_arrow
question_answer 150) The smallest angiosperm flower is
A)
Wolffia
done
clear
B)
Ranunculus
done
clear
C)
Rafflesia
done
clear
D)
Stellaria
done
clear
View Answer play_arrow
question_answer 151) The pyramid of energy is always
A)
opaque
done
clear
B)
horizontal
done
clear
C)
upright
done
clear
D)
inverted
done
clear
View Answer play_arrow
question_answer 152) The transition zone between the two vegetations of ecosystem is called
A)
ecotone
done
clear
B)
ecocline
done
clear
C)
ecosystem
done
clear
D)
ecesis
done
clear
View Answer play_arrow
question_answer 153) Thermoregulatory centre of human body is associated with
A)
cerebrum
done
clear
B)
cerebellum
done
clear
C)
hypothalamus
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 154) Body cavity of adult Ascaris is
A)
haemocoel
done
clear
B)
amphicoel
done
clear
C)
pseudocoel
done
clear
D)
schizocoel
done
clear
View Answer play_arrow
question_answer 155) Cottar cells are characteristic of
A)
earthworm
done
clear
B)
roundworms
done
clear
C)
coelenterata
done
clear
D)
sponges
done
clear
View Answer play_arrow
question_answer 156) In honey bee, the drones are
A)
sterile male
done
clear
B)
fertile male
done
clear
C)
fertile female
done
clear
D)
sterile female
done
clear
View Answer play_arrow
question_answer 157) Plasmids are found in
A)
virus
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
viroid
done
clear
View Answer play_arrow
question_answer 158) Blood leaving the liver and going towards heart is rich in
A)
bile
done
clear
B)
urea
done
clear
C)
ammonia
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 159) Membrane that covers the vacuole in a plant cell is called
A)
tonoplast
done
clear
B)
tonoplasm
done
clear
C)
jacket
done
clear
D)
cell membrane
done
clear
View Answer play_arrow
question_answer 160) In earthworm, gizzard is found, in which of the following segments?
A)
9th segment
done
clear
B)
18th segment
done
clear
C)
13th segment
done
clear
D)
16th segment
done
clear
View Answer play_arrow
question_answer 161) The infective stage of Entamoeba histolytica is
A)
trophozoite stage
done
clear
B)
binucleated cyst stage
done
clear
C)
tetranucleated cyst stage
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 162) The initiation codon in eukaryotes is
A)
AUG
done
clear
B)
UGA
done
clear
C)
UAG
done
clear
D)
UAA
done
clear
View Answer play_arrow
question_answer 163) The number of heart chambers found in cockroach is
A)
4
done
clear
B)
7
done
clear
C)
5
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 164) % sign is used for
A)
actinomorphic flower
done
clear
B)
zygomorphic flower
done
clear
C)
incomplete flower
done
clear
D)
epigynous flower
done
clear
View Answer play_arrow
question_answer 165) Nuclear membrane is continuous with
A)
rough endoplasmic reticulum
done
clear
B)
smooth endoplasmic reticulum
done
clear
C)
cell membrane
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 166) XO chromosomal abnormality in humans causes
A)
Turners syndrome
done
clear
B)
Downs syndrome
done
clear
C)
Darwins syndrome
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 167) Fertilization of ovum takes place in rabbit, man and other placenta! mammals in
A)
ovary
done
clear
B)
fallopian tube
done
clear
C)
cervix
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 168) At what stage in test tube babies, the zygote is implanted in human female?
A)
32-celled stage
done
clear
B)
64-celled stage
done
clear
C)
100-celled stage
done
clear
D)
164-celled stage
done
clear
View Answer play_arrow
question_answer 169) Pentoses and hexoses are common
A)
monosaccharides
done
clear
B)
disaccharides
done
clear
C)
polysaccharides
done
clear
D)
oligosaccharides
done
clear
View Answer play_arrow
question_answer 170) Pheromone is
A)
a product of endocrine gland
done
clear
B)
used for animal communication
done
clear
C)
messenger RNA
done
clear
D)
always protein
done
clear
View Answer play_arrow
question_answer 171) Secretion is under control of neurosecretory nerve axons in
A)
pineal gland
done
clear
B)
adrenal cortex
done
clear
C)
anterior pituitary
done
clear
D)
posterior pituitary
done
clear
View Answer play_arrow
question_answer 172) The smallest endocrine gland is
A)
thyroid
done
clear
B)
parathyroid
done
clear
C)
pituitary
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 173) Gland responsible for calcium metabolism!
A)
thymus
done
clear
B)
thyroid
done
clear
C)
parathyroid
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 174) The daughter born to haemophilic father and normal mother could be
A)
normal
done
clear
B)
carrier
done
clear
C)
haemophilic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 175) Mast cells secrete
A)
serotonin
done
clear
B)
heparin
done
clear
C)
histamine
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 176) Role of spleen in mammal is
A)
to control blood pressure
done
clear
B)
to assist liver
done
clear
C)
to act as haemopoietic tissue
done
clear
D)
to assist kidneys
done
clear
View Answer play_arrow
question_answer 177) Excretory product of spider is
A)
uric acid
done
clear
B)
ammonia
done
clear
C)
guanine
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 178) Green glands present in some arthropods help in
A)
respiration
done
clear
B)
excretion
done
clear
C)
digestion
done
clear
D)
reproduction
done
clear
View Answer play_arrow
question_answer 179) Right lung of rabbit is divided into
A)
four lobes
done
clear
B)
two lobes
done
clear
C)
six lobes
done
clear
D)
eight lobes
done
clear
View Answer play_arrow
question_answer 180) Haemoglobin is having maximum affinity with
A)
\[C{{O}_{2}}\]
done
clear
B)
\[CO\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow