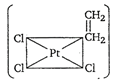
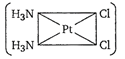
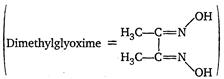question_answer 1) The total number of possible isomers for the complex compound \[[C{{u}^{II}}{{(N{{H}_{3}})}_{4}}][P{{t}^{II}}C{{l}_{4}}]\] [AIPMT 1998]
A)
3
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 2) IUPAC name of \[[Pt\,{{(N{{H}_{3}})}_{3}}\,(Br)\,(N{{O}_{2}})\,Cl]\,Ct\] is [AIPMT 1998]
A)
Triammine chlorobromonitro platinum (IV) chloride
done
clear
B)
Triammine bromonitrochloro platinum (IV) chloride
done
clear
C)
Triammine bromochloronitro platinum (IV) chloride
done
clear
D)
Triammine nitrochlorobromo platinum (IV) chloride
done
clear
View Answer play_arrow
question_answer 3)
A co-ordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three mole ions in an aqueous solution. On reacting this solution with excess of \[AgN{{O}_{3}}\] solution, we get two moles of \[AgCl\] precipitate. The ionic formula for this complex would be : [AIPMT 1998]
A)
\[[Co{{(N{{H}_{3}})}_{4}}(N{{O}_{2}})Cl]\,[(N{{H}_{3}}Cl]\]
done
clear
B)
\[[Co\,(N{{H}_{3}})Cl]\,[Cl(N{{O}_{2}})]\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}]\,[{{(N{{O}_{2}})}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 4) The correct structure of \[Fe{{(CO)}_{5}}\] is : [AIPMT 2000]
A)
trigonal bipyramidal
done
clear
B)
octahedral
done
clear
C)
tetrahedral
done
clear
D)
square pyramidal
done
clear
View Answer play_arrow
question_answer 5) Which one of the following complexes will have four different isomers? [AIPMT 2000]
A)
\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
done
clear
B)
\[[Co(en)(N{{H}_{3}})C{{l}_{2}}]Cl\]
done
clear
C)
\[[Co{{(PP{{H}_{3}})}_{2}}(N{{H}_{3}})C{{l}_{2}}]Cl\]
done
clear
D)
\[[Co{{(en)}_{3}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 6) In the separation of \[C{{u}^{2+}}\] and \[C{{d}^{2+}}\] in 2nd group of qualitative analysis of cations, tetramine copper (II) sulphate and tetrammine cadmium (II) sulphate react with KCN to form the corresponding cyano complexes, which one of the following pairs of the complexes and their relative stability enables the separation of \[C{{u}^{2+}}\] and \[C{{d}^{2+}}\]? [AIPMT 2000]
A)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\] : less stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : more stable
done
clear
B)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\] : more stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : less stable
done
clear
C)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\] : less stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : more stable
done
clear
D)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\] : more stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : less stable
done
clear
View Answer play_arrow
question_answer 7) Which one of the following will give maximum number of isomers? [AIPMT 2001]
A)
\[[Co\,(N{{H}_{3}})C{{l}_{2}}]\]
done
clear
B)
\[{{[Ni\,(en)\,\,{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
C)
\[{{[Ni\,({{C}_{2}}{{O}_{4}})\,{{(en)}_{2}}]}^{2-}}\]
done
clear
D)
\[{{[Cr{{(SCN)}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]
done
clear
View Answer play_arrow
question_answer 8) Coordination number of \[Ni\] in \[{{[Ni{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{4-}}\] is: [AIPMT 2001]
A)
3
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 9) Which of the following organometallic compound has \[\sigma \] and \[\pi \] bond? [AIPMT 2001]
A)
\[[Fe({{\eta }^{5}}-{{C}_{5}}{{H}_{5}}){{ & }_{2}}]\]
done
clear
B)
\[K[PtC{{l}_{3}}({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
C)
\[{{[Co{{(CO)}_{5}}N{{H}_{3}}]}^{2+}}\]
done
clear
D)
\[Fe{{(C{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 10) Which statements is incorrect? [AIPMT 2001]
A)
\[Ni{{(CO)}_{4}}\]-Tetrahedral, paramagnetic
done
clear
B)
\[Ni(CN)_{4}^{2-}\]-Square planar, diamagnetic
done
clear
C)
\[Ni{{(CO)}_{4}}\]-Tetrahedral, diamagnetic
done
clear
D)
\[{{[Ni\,{{(Cl)}_{4}}]}^{2}}\]-Tetrahedral, paramagnetic
done
clear
View Answer play_arrow
question_answer 11) Which of die following will exhibit maximum ionic conductivity? [AIPMT 2001]
A)
\[{{K}_{4}}[Fe\,{{(CN)}_{6}}]\]
done
clear
B)
\[[Co\,{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
C)
\[[Cu{{(N{{H}_{3}})}_{4}}3C{{l}_{2}}\]
done
clear
D)
\[[Ni\,{{(CO)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 12) Atomic number of Cr and Fe are respectively 24 and 26, which of the following is paramagnetic with the spin of electron: [AIPMT 2002]
A)
\[[Cr\text{ }{{(CO)}_{6}}]\]
done
clear
B)
\[[Fe\text{ }{{(CO)}_{5}}]\]
done
clear
C)
\[{{[Fe\text{ }{{(CN)}_{6}}]}^{4\,-}}\]
done
clear
D)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 13) The hypothetical complex chloro diaquatriammine cobalt (III) chloride can be represented as: [AIPMT 2002]
A)
\[[CoCl{{(N{{H}_{3}})}_{3}}{{({{H}_{2}}O)}_{2}}]C{{l}_{2}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{3}}({{H}_{2}}O)C{{l}_{3}}]\]
done
clear
C)
\[[Co{{(N{{H}_{2}})}_{3}}{{({{H}_{2}}O)}_{2}}Cl]\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{3}}{{({{H}_{2}}O)}_{3}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 14) In the silver plating of copper,\[K[Ag{{(CN)}_{2}}]\] is used instead of \[AgN{{O}_{3}}\]. The reason is: [AIPMT 2002]
A)
a thin layer of Ag is formed on Cu
done
clear
B)
more voltage is required
done
clear
C)
\[A{{g}^{+}}\] ions are completely removed from solution
done
clear
D)
less availability of \[A{{g}^{+}}\] ions, as Cu cannot displace Ag from \[{{[Ag\,{{(CN)}_{2}}]}^{-}}\] ion
done
clear
View Answer play_arrow
question_answer 15) \[CuS{{O}_{4}}\] when reacts with KCN forms CuCN. Which is insoluble in water. It is soluble in excess of KCN, due to formation of the following complex: [AIPMT 2002]
A)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\]
done
clear
B)
\[[{{K}_{3}}\,{{(CuCN)}_{4}}]\]
done
clear
C)
\[CuC{{N}_{2}}\]
done
clear
D)
\[Cu[K\text{ }Cu{{(CN)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 16) Which one of the following octahedral complexes will not show geometrical isomerism? (A and B are monodentate ligands) [AIPMT 2003]
A)
\[[M{{A}_{4}}{{B}_{2}}]\]
done
clear
B)
\[[M{{A}_{5}}B]\]
done
clear
C)
\[[M{{A}_{2}}{{B}_{4}}]\]
done
clear
D)
\[[M{{A}_{3}}{{B}_{3}}]\]
done
clear
View Answer play_arrow
question_answer 17) According to IUPAC nomenclature sodium nitroprusside is named as: [AIPMT 2003]
A)
sodium pentacyanonitrosyl ferrate (II)
done
clear
B)
sodium pentacyanonitrosyl ferrate (III)
done
clear
C)
sodium nitroferricyanide
done
clear
D)
sodium nitroferrocyanide
done
clear
View Answer play_arrow
question_answer 18) Among the following, which is not the \[\pi \]-bonded organometallic compound? [AIPMT 2003]
A)
\[Cr{{({{\eta }^{6}}-{{C}_{6}}{{H}_{6}})}_{2}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{4}}Sn\]
done
clear
C)
\[K[PtC{{l}_{3}}\,({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
D)
\[Fe{{({{\eta }^{5}}-{{C}_{5}}{{H}_{5}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 19)
The number of unpaired electrons in me complex ion \[{{[Co{{F}_{6}}]}^{3-}}\] is: [AIPMT 2003] (Atomic number \[Co=27\])
A)
4
done
clear
B)
zero
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 20) Which of the following is considered to be ananticancer species? [AIPMT (S) 2004]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 21) Which of the following co-ordination compounds would exhibit optical isomerism? [AIPMT (S) 2004]
A)
Pentaamminenitrocobalt\[\text{(III)}\] iodide
done
clear
B)
Diamminedinitroplatinum\[\text{(II)}\]
done
clear
C)
traos-dicyanobis (ethylenediamine)
done
clear
D)
Tris-(ethylendiamine) cobalt \[\text{(III)}\] bromide
done
clear
View Answer play_arrow
question_answer 22) Among \[{{[Ni{{(CO)}_{4}}]}^{2-}},{{[Ni{{(CN)}_{4}}]}^{2-}},\]\[{{[NiC{{l}_{4}}]}^{2-}}\]species, the hybridisation states of the Ni atom are, respectively: (At. no. of Ni = 28) [AIPMT (S) 2004]
A)
\[s{{p}^{3}},ds{{p}^{2}}\,\,ds{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}},ds{{p}^{2}}\,\,s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}},s{{p}^{3}}\,\,ds{{p}^{2}}\]
done
clear
D)
\[ds{{p}^{2}},s{{p}^{3}}\,\,sp\]
done
clear
View Answer play_arrow
question_answer 23) \[C{{N}^{-}}\]is strong field ligand. This is due to the fact that: [AIPMT (S) 2004]
A)
it carries negative charge
done
clear
B)
it is a pseudo halide
done
clear
C)
it can accept electrons from metal species
done
clear
D)
it forms high spin complexes with metal species
done
clear
View Answer play_arrow
question_answer 24) Considering \[{{H}_{2}}O\]as weak field ligand, the number of unpaired electrons in\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]will be (At. no. of \[Mn=25\]): [AIPMT (S) 2004]
A)
three
done
clear
B)
five
done
clear
C)
two
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 25) Which of the following does not have a metal-carbon bond? [AIPMT (S) 2004]
A)
\[Al{{(O{{C}_{2}}{{H}_{5}})}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}MgBr\]
done
clear
C)
\[K[Pt({{C}_{2}}{{H}_{4}})C{{l}_{3}}]\]
done
clear
D)
\[Ni{{(CO)}_{4}}\]
done
clear
View Answer play_arrow
question_answer 26) In an octahedral structure, the pair of dorbitals involved in \[{{d}^{2}}s{{p}^{3}}\]hybridisation is: [AIPMT (S) 2004]
A)
\[{{d}_{{{x}^{2}}-{{y}^{2}}}}{{d}_{{{z}^{2}}}}\]
done
clear
B)
\[{{d}_{xz}},{{d}_{{{x}^{2}}-{{y}^{2}}}}\]
done
clear
C)
\[{{d}_{{{z}^{2}}'}}{{d}_{xz}}\]
done
clear
D)
\[{{d}_{xy}},{{d}_{yz}}\]
done
clear
View Answer play_arrow
question_answer 27)
Which one of the following is an inner orbital complex as well as diamagnetic in behaviour? [AIPMT (S) 2005] (Atomic no. : Zn = 30, Cr=24, Co=27, Ni = 28)
A)
\[{{[Zn\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Ni\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 28) Which one of the following is expected to exhibit optical isomerism? (en = ethylenediamine)
A)
cis-[Pt (NH3)2 Cl2]
done
clear
B)
trans-[Co(en)2 Cl2]
done
clear
C)
trans-[Pt (NH3)2 Cl2]
done
clear
D)
cis -[Co (en)2Cl2]
done
clear
View Answer play_arrow
question_answer 29) Copper sulphate dissolves in excess of KCN to give: [AIPMT (S) 2006]
A)
CuCN
done
clear
B)
\[{{[Cu{{(CN)}_{4}}]}^{3-}}\]
done
clear
C)
\[{{[Cu{{(CN)}_{4}}]}^{2-}}\]
done
clear
D)
\[Cu\,{{(CN)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 30) [NH(CH2) NHCO (CH2)4 CO]n is a: [AIPMT (S) 2006]
A)
co-polymer
done
clear
B)
addition polymer
done
clear
C)
thermo-setting polymer
done
clear
D)
homopolymer
done
clear
View Answer play_arrow
question_answer 31) [Co (NH3)4 (NO 2)2]CI exhibit its: [AIPMT (S) 2006]
A)
linkage isomerism, ionization isomerism and optical isomerism
done
clear
B)
linkage isomerism, ionization isomerism and geometrical isomerism
done
clear
C)
ionization isomerism, geometrical isomerism and optical isomerism
done
clear
D)
linkage isomerism, geometrical isomerism and optical isomerism
done
clear
View Answer play_arrow
question_answer 32) \[[Cr{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\] (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in die chromium of the complex is : [AIPMT (S) 2006]
A)
\[3d_{{{x}^{2}}-{{y}^{2}}}^{1},\,3d_{{{z}^{2}}}^{1},\,3d_{xz}^{1}\]
done
clear
B)
\[3d_{xy}^{1},\,3d_{{{x}^{2}}-{{y}^{2}}}^{1},\,3d_{yz}^{1}\]
done
clear
C)
\[3d_{xy}^{1},\,3d_{zy}^{1},\,3d_{xz}^{1}\]
done
clear
D)
\[3d_{xy}^{1},\,3d_{yz}^{1},\,3d_{{{z}^{2}}}^{1}\]
done
clear
View Answer play_arrow
question_answer 33)
Which of the following will give a pair of enantiomorphs? [AIPMT (S) 2007] (en = NH2 CH2 CH2 NH2)
A)
[Co(NH3)4Cl2]NO2
done
clear
B)
[Cr(NH3)6] [Co(CN)6]
done
clear
C)
[Co(en)2Cl2]Cl
done
clear
D)
[Pt(NH3)4] [PtCl6]
done
clear
View Answer play_arrow
question_answer 34)
The d-electron configurations of Cr2+, Mn2+, Fe2+ and Ni2+ are 3d4, 3d5, 3d6 and 3d8 respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour? [AIPMT (S) 2007] (At. no. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
A)
[Mn(H2O)6]2+
done
clear
B)
[Fe(H2O)6]2+
done
clear
C)
[Ni(H2O)6]2+
done
clear
D)
[Cr(H2O)6]2+
done
clear
View Answer play_arrow
question_answer 35)
Which of the following complexes exhibits the highest paramagnetic behaviour? [AIPMT (S) 2008] where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities (At no : Ti = 22, V = 23, Fe = 26, Co = 27)
A)
\[{{[V{{(gly)}_{2}}{{(OH)}_{2}}{{(N{{H}_{3}})}_{2}}]}^{+}}\]
done
clear
B)
\[{{[Fe(en)(bpy){{(N{{H}_{3}})}_{2}}]}^{2+}}\]
done
clear
C)
\[{{[Co{{(OX)}_{2}}{{(OH)}_{2}}]}^{-}}\]
done
clear
D)
\[{{[Ti{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 36) In which of the following coordination entities the magnitude of \[{{\Delta }_{o}}\](CCFSE in octahedral field) will be maximum? (Atomic number Co = 27) [AIPMT (S) 2008]
A)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[Co{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 37) Which of the following does not show optical isomerism? (en = ethylenediamine) [AIPMT (S) 2009]
A)
\[\text{1g}{{\text{m}}^{\text{-1}}}.\]
done
clear
B)
\[\frac{4}{3}M{{l}^{2}}\]
done
clear
C)
\[\frac{2}{3}M{{l}^{2}}\]
done
clear
D)
\[\frac{13}{3}M{{l}^{2}}\]
done
clear
View Answer play_arrow
question_answer 38)
Which of the following complex ions is expected to absorb visible light? [AIPMT (S) 2009] \[1m{{s}^{-2}}.\]
A)
\[2\sqrt{10}kg\]
done
clear
B)
\[10\sqrt{2}kg\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}B\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 39) Which of the following complex ions is not expected to absorb visible light? [AIPMT (S) 2010]
A)
\[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\]
done
clear
B)
\[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
done
clear
C)
\[{{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
D)
\[{{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
View Answer play_arrow
question_answer 40) Crystal field stabilization energy for high spin d4 octahedral complex is [AIPMT (S) 2010]
A)
\[-1.8{{\Delta }_{o}}\]
done
clear
B)
\[-1.6{{\Delta }_{o}}+P\]
done
clear
C)
\[-1.2{{\Delta }_{o}}\]
done
clear
D)
\[-0.6{{\Delta }_{o}}\]
done
clear
View Answer play_arrow
question_answer 41) Crystal field stabilization energy for high spin d4 octahedral complex is [AIPMT (S) 2010]
A)
\[-1.8{{\Delta }_{o}}\]
done
clear
B)
\[-1.6{{\Delta }_{o}}+P\]
done
clear
C)
\[-1.2{{\Delta }_{o}}\]
done
clear
D)
\[-0.6{{\Delta }_{o}}\]
done
clear
View Answer play_arrow
question_answer 42) The existence of two different coloured complexes with the composition of \[{{[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]}^{+}}\] is due to [AIPMT (S) 2010]
A)
linkage isomerism
done
clear
B)
geometrical isomerism
done
clear
C)
coordination isomerism
done
clear
D)
ionisation isomerism
done
clear
View Answer play_arrow
question_answer 43) Which one of the following complexes is not expected to exhibit isomerism? [AIPMT (M) 2010]
A)
\[{{[Ni{{(N{{H}_{3}})}_{4}}{{({{H}_{2}}O)}_{2}}]}^{2+}}\]
done
clear
B)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
C)
\[[Ni{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
D)
\[{{[Ni{{(en)}_{3}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 44)
The d-electron configurations of \[C{{r}^{2+}},M{{n}^{2+}},\] \[F{{e}^{2+}}\] and \[C{{o}^{2+}}\] are \[{{d}^{4}},{{d}^{5}},{{d}^{6}}\] and \[{{d}^{7}}\] respectively.[AIPMT (S) 2011] Which one of the following will exhibit minimum paramagnetic behaviour? (At. no. Cr = 24, Mn = 25, Fe = 26, Co = 27)
A)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 45) Of the following complex ions, which is diamagnetic in nature? [AIPMT (S) 2011]
A)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
B)
\[{{[CuC{{l}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Co{{F}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 46) The complexes \[[Co{{(N{{H}_{3}})}_{6}}][R{{(CN)}_{6}}]\] and \[[Cr{{(N{{H}_{3}})}_{6}}][Co{{(CN)}_{6}}]\] are the examples of which type of isomerism? [AIPMT (S) 2011]
A)
Ionisation isomerism
done
clear
B)
Co-ordination isomerism
done
clear
C)
Geometrical isomerism
done
clear
D)
Linkage isomerism
done
clear
View Answer play_arrow
question_answer 47) The complex,\[[Pt(Py)(N{{H}_{3}})BrCl]\]will have how many geometrical isomers? [AIPMT (S) 2011]
A)
4
done
clear
B)
0
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 48) Which of the following carbonyls will have the strongest CO bond? [AIPMT (M) 2011]
A)
\[\text{Mn(CO)}_{\text{6}}^{\text{+}}\]
done
clear
B)
\[\text{Cr(CO)}_{\text{6}}^{{}}\]
done
clear
C)
\[\text{V(CO)}_{\text{6}}^{-}\]
done
clear
D)
\[\text{Fe(CO)}_{5}^{{}}\]
done
clear
View Answer play_arrow
question_answer 49)
Which of the following complex compounds will exhibit highest paramagnetic behaviour? [AIPMT (M) 2011] \[(At.\,no.:Ti=22,Cr=24,Co=27,Zn=30)\]
A)
\[{{[Ti{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 50) Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour? [AIPMT (S) 2012]
A)
\[{{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
B)
\[{{\text{ }\!\![\!\!\text{ Zn(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
C)
\[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
done
clear
D)
\[{{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
done
clear
View Answer play_arrow
question_answer 51) Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammonia cal Ni(II). Which of the following statements is not true? [AIPMT (M) 2012]
A)
Red complex has a square planar geometry
done
clear
B)
Complex has symmetrical H-bonding
done
clear
C)
Red complex has a tetrahedral geometry
done
clear
D)
Dimethylglyoxime functions as bin dentate ligand
done
clear
View Answer play_arrow
question_answer 52)
Low spin complex of d6cation in an octahedral field will have the following energy [AIPMT (M) 2012] (\[{{\Delta }_{o}}=\] crystal field splitting energy in an octahedral field, P = electron pairing energy)
A)
\[\frac{-12}{5}{{\Delta }_{o}}+p\]
done
clear
B)
\[\frac{-12}{5}{{\Delta }_{o}}+3p\]
done
clear
C)
\[\frac{-2}{5}{{\Delta }_{o}}+2p\]
done
clear
D)
\[\frac{-2}{5}{{\Delta }_{o}}+p\]
done
clear
View Answer play_arrow
question_answer 53) A magnetic moment of 1.73 BM will be shown by one among the following [NEET 2013]
A)
\[{{\text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
done
clear
B)
\[{{\text{ }\!\![\!\!\text{ (NiCN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\]
done
clear
C)
\[\text{TiC}{{\text{l}}_{\text{4}}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 54) Among the following complexes, the one which shows zero crystal field stabilization energy (CFSE) is [AIPMT 2014]
A)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}},\]
done
clear
D)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following complexes is used to be as an anticancer agent? [AIPMT 2014]
A)
\[mer-[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
B)
\[cis-[PtC{{l}_{2}}{{(N{{H}_{3}})}_{2}}]\]
done
clear
C)
\[cis-{{K}_{2}}[PtC{{l}_{2}}B{{r}_{2}}]\]
done
clear
D)
\[N{{a}_{2}}CoC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 56) Cobalt (III) chloride forms several octahedral complexes with ammonia. Which of the following will not give test for chloride ions with silver nitrate at 25°C? [NEET 2015 ]
A)
\[CoC{{l}_{3}}.3N{{H}_{3}}\]
done
clear
B)
\[CoC{{l}_{3}}.\,4N{{H}_{3}}\]
done
clear
C)
\[CoC{{l}_{3}}.5N{{H}_{3}}\]
done
clear
D)
\[CoC{{l}_{3}}.6N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of these statements about \[{{[Co{{(CN)}_{6}}]}^{3-}}\] is true? [NEET 2015 ]
A)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\]has no unpaired electrons and will be in a low-spin configuration.
done
clear
B)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\] has four unpaired electrons and will be in a low-spin configuration.
done
clear
C)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\] has four unpaired electrons and will be in a high-spin configuration.
done
clear
D)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\] has no unpaired electrons and will be in a high-spin configuration.
done
clear
View Answer play_arrow
question_answer 58) Number of possible isomers for the complex \[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\] will be (en = ethylenediamine) [NEET 2015 (Re)]
A)
2
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 59) The hybridisation involved in complex \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]is (Atomic number of Ni = 28) [NEET 2015 (Re)]
A)
\[ds{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[{{d}^{2}}s{{p}^{2}}\]
done
clear
D)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 60)
The sum of coordination number and oxidation number of the metal M in the complex
A)
9
done
clear
B)
6
done
clear
C)
7
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 61) The name of complex ion, \[{{[Fe{{(CN)}_{6}}]}^{3-}}\] is [NEET 2015 (Re)]
A)
hexacyanoiron (III) ion
done
clear
B)
hexacyanitoferrate (III) ion
done
clear
C)
tricyanoferrate (III) ion
done
clear
D)
hexacyanidoferrate (lll)ion
done
clear
View Answer play_arrow
question_answer 62) Which of the following has longest C-O bond length? (Free C-O bond length in Co is\[1.128\overset{\text{o}}{\mathop{\text{A}}}\,\]). [NEET - 2016]
A)
\[Ni{{(CO)}_{4}}\]
done
clear
B)
\[{{[Co{{(CO)}_{4}}]}^{\text{O}-}}\]
done
clear
C)
\[{{[Fe{{(CO)}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[Mn{{(CO)}_{6}}]}^{+}}\]
done
clear
View Answer play_arrow
question_answer 63) Correct increasing order for the wavelengths of absorption in the visible region for the complexes of \[C{{o}^{3+}}\]is [NEET-2017]
A)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}},{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{(N{{H}_{3}}O)}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(e{{n}_{3}})}_{3}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 64) Pick out the correct statement with respect \[{{[Mn{{(CN)}_{6}}]}^{3-}}\] [NEET-2017]
A)
It is \[ds{{p}^{2}}\]hybridised and square planar
done
clear
B)
It is \[s{{p}^{3}}{{d}^{2}}\]hybridised and octahedral
done
clear
C)
It is \[s{{p}^{3}}{{d}^{2}}\] hybridised and tetrahedral
done
clear
D)
It is\[{{d}^{2}}s{{p}^{3}}\] hybridised and octahedral
done
clear
View Answer play_arrow
question_answer 65) The correct order of the stoichiometries of AgCl formed when\[AgN{{O}_{3}}\]in excess is treated with the complexes : \[CoC{{l}_{3}}.6N{{H}_{3}},CoC{{l}_{3}}.5N{{H}_{3}},CoC{{l}_{3}}.4N{{H}_{3}}\] respectively is [NEET-2017]
A)
\[2\,AgCl,3AgCl,\,1\,AgCl\]
done
clear
B)
\[1\,AgCl,3AgCl,\,2\,AgCl\]
done
clear
C)
\[3\,AgCl,1AgCl,\,2\,AgCl\]
done
clear
D)
\[3\,AgCl,2AgCl,\,1\,AgCl\]
done
clear
View Answer play_arrow
question_answer 66) Iron carbonyl, \[\text{Fe(CO}{{\text{)}}_{\text{5}}}\] is [NEET - 2018]
A)
Trinuclear
done
clear
B)
Mononuclear
done
clear
C)
Tetranuclear
done
clear
D)
Dinuclear
done
clear
View Answer play_arrow
question_answer 67) The type of isomerism shown by the complex \[\text{ }\!\![\!\!\text{ CoC}{{\text{l}}_{\text{2}}}{{\text{(en)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }\] is [NEET - 2018]
A)
Ionization isomerism
done
clear
B)
Coordination isomerism
done
clear
C)
Geometrical isomerism
done
clear
D)
Linkage isomerism
done
clear
View Answer play_arrow
question_answer 68) The geometry and magnetic behaviour of the complex \[\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }\] are [NEET - 2018]
A)
Square planar geometry and paramagnetic
done
clear
B)
Tetrahedral geometry and diamagnetic
done
clear
C)
Square planar geometry and diamagnetic
done
clear
D)
Tetrahedral geometry and paramagnetic
done
clear
View Answer play_arrow
question_answer 69) Which of the following species is not stable? [NEET 2019]
A)
\[{{\left[ Sn{{(OH)}_{6}} \right]}^{2}}\]
done
clear
B)
\[{{\left[ SiC{{l}_{6}} \right]}^{2}}\]
done
clear
C)
\[{{\left[ Si{{F}_{6}} \right]}^{2}}\]
done
clear
D)
\[{{\left[ GeC{{l}_{6}} \right]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 70) What is the correct electronic configuration of the central atom in \[{{K}_{4}}\left[ Fe{{(CN)}_{6}} \right]\]based on crystal field theory? [NEET 2019]
A)
\[{{e}^{3}}t_{2}^{3}\]
done
clear
B)
\[{{e}^{4}}t_{2}^{2}\]
done
clear
C)
\[t_{2g}^{4}e_{g}^{2}\]
done
clear
D)
\[t_{2g}^{6}e_{g}^{0}\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is the correct order of increasing field strength of ligands to form coordination compounds? [NEET 2020]
A)
\[SC{{N}^{-}}<{{F}^{-}}<C{{N}^{-}}<{{C}_{2}}O_{4}^{2-}\]
done
clear
B)
\[{{F}^{-}}<SC{{N}^{-}}<{{C}_{2}}O_{4}^{2-}<C{{N}^{-}}\]
done
clear
C)
\[C{{N}^{-}}<{{C}_{2}}O_{4}^{2-}<SC{{N}^{-}}<{{F}^{-}}\]
done
clear
D)
\[SC{{N}^{-}}<{{F}^{-}}<{{C}_{2}}O_{4}^{2-}<C{{N}^{-}}\]
done
clear
View Answer play_arrow