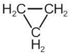
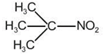
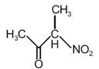
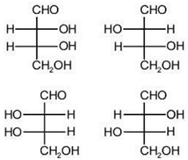
question_answer 1) Planck's constant (h), speed of light in vacuum \[\text{(c)}\] and Newton's gravitational constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length?
A)
\[\frac{\sqrt{hc}}{{{c}^{\frac{3}{2}}}}\]
done
clear
B)
\[\frac{\sqrt{hc}}{{{c}^{\frac{5}{2}}}}\]
done
clear
C)
\[\sqrt{\frac{hc}{G}}\]
done
clear
D)
\[\sqrt{\frac{Gc}{{{h}^{\frac{3}{2}}}}}\]
done
clear
View Answer play_arrow
question_answer 2) Two cars P and Q start from a point at the same time in a straight line and their positions are represented by \[{{X}_{p}}(t)=at+b{{t}^{2}}and\,{{X}_{Q}}(t)=ft-{{t}^{2}}\]. At what time do the cars have the same velocity?
A)
\[\frac{a-f}{1+b}\]
done
clear
B)
\[\frac{a+f}{2(b-1)}\]
done
clear
C)
\[\frac{a+f}{2(1+b)}\]
done
clear
D)
\[\frac{f-a}{2(1+b)}\]
done
clear
View Answer play_arrow
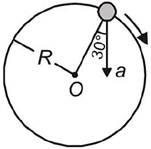
question_answer 3)
In the given figure,\[a=15m/{{s}^{2}}\]represents the total acceleration of a particle moving in the clockwise direction in a circle of radius \[R=2.5\]m at a given instant of time. The speed of the particle is
A)
4.5 m/s
done
clear
B)
5.0 m/s
done
clear
C)
5.7 m/s
done
clear
D)
6.2 m/s
done
clear
View Answer play_arrow
question_answer 4)
A rigid ball of mass m strikes a rigid wall at \[{{60}^{{}^\circ }}\] and gets reflected without loss of speed as shown in the figure below. The value of impulse imparted by the wall on the ball will be
A)
mV
done
clear
B)
2mV
done
clear
C)
\[\frac{mV}{2}\]
done
clear
D)
\[\frac{mV}{3}\]
done
clear
View Answer play_arrow
question_answer 5) A bullet of mass 10 g moving horizontally with a velocity of\[400m{{s}^{-1}}\]strikes a wood block of mass 2 kg which is suspended by light inextensible string of length 5 m. As a result, the centre of gravity of the block found to rise a vertical distance of 10 cm. The speed of the bullet after it emerges out horizontally from the block will be
A)
\[100m{{s}^{-1}}\]
done
clear
B)
\[80m{{s}^{-1}}\]
done
clear
C)
\[120m{{s}^{-1}}\]
done
clear
D)
\[160m{{s}^{-1}}\]
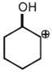
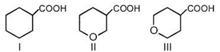
done
clear
View Answer play_arrow
question_answer 6) Two identical balls A and B having velocities of 0.5 m/s and -0.3 m/s respectively collide elastically in one dimension. The velocities of B and A after the collision respectively will be
A)
- 0.5 m/s and 0.3 m/s
done
clear
B)
0.5 m/s and - 0.3 m/s
done
clear
C)
- 0.3 m/s and 0.5 m/s
done
clear
D)
0.3 m/s and 0.5 m/s
done
clear
View Answer play_arrow
question_answer 7) A particle moves from a point \[(-2\hat{i}+5\hat{j})to(4\hat{j}+3\hat{k})\] when a force of\[(4\hat{i}+3\hat{j})N\] is applied. How much work has been done by the force?
A)
8 J
done
clear
B)
11 J
done
clear
C)
5 J
done
clear
D)
2 J
done
clear
View Answer play_arrow
question_answer 8) Two rotating bodies A and B of masses m and 2m with moments of inertia\[{{I}_{A}}\,and\,{{I}_{g}}({{I}_{g}}>{{I}_{A}})\]have equal kinetic energy of rotation. If \[{{L}_{A}}and\text{ }{{L}_{B}}\]be their angular momenta respectively, then
A)
\[{{L}_{A}}=\frac{{{L}_{B}}}{2}\]
done
clear
B)
\[{{L}_{A}}=2{{L}_{B}}\]
done
clear
C)
\[{{L}_{g}}>{{L}_{A}}\]
done
clear
D)
\[{{L}_{A}}>{{L}_{g}}\]
done
clear
View Answer play_arrow
question_answer 9) A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of the same mass A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of the same mass and same radius is also rotating about its geometrical axis with an angular speed twice that of the sphere. The ratio of their kinetic energies of rotation \[\left( \frac{{{E}_{sphere}}}{{{E}_{cylinder}}} \right)\] will be.
A)
2:3
done
clear
B)
1:5
done
clear
C)
1:4
done
clear
D)
3:1
done
clear
View Answer play_arrow
question_answer 10) A light rod of length l has two masses \[{{m}_{1}}\]and \[{{m}_{2}}\] attached to its two ends. The moment of inertia of the system about an axis perpendicular to the rod and passing through the centre of mass is
A)
\[\frac{{{m}_{1}}{{m}_{2}}}{{{m}_{1}}+{{m}_{2}}}{{I}^{2}}\]
done
clear
B)
\[\frac{{{m}_{1}}+{{m}_{2}}}{{{m}_{1}}{{m}_{2}}}{{I}^{2}}\]
done
clear
C)
\[({{m}_{1}}+{{m}_{2}}){{I}^{2}}\]
done
clear
D)
\[\sqrt{{{m}_{1}}{{m}_{2}}}{{I}^{2}}\]
done
clear
View Answer play_arrow
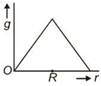
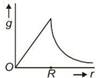
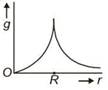
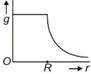
question_answer 11) Starting from the centre of the earth having radius R, the variation of g (acceleration due to gravity) is shown by
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 12) A satellite of mass m is orbiting the earth (of radius R) at a height h from its surface. The total energy of the satellite in terms of\[{{g}_{0}}\],the value of acceleration due to gravity at the earth's surface, is
A)
\[\frac{m{{g}_{o}}{{R}^{2}}}{2(R+h)}\]
done
clear
B)
\[\frac{m{{g}_{o}}{{R}^{2}}}{2(R+h)}\]
done
clear
C)
\[\frac{2{{m}_{o}}{{R}^{2}}}{R+h}\]
done
clear
D)
\[\frac{2{{m}_{o}}{{R}^{2}}}{R+h}\]
done
clear
View Answer play_arrow
question_answer 13) A rectangular film of liquid is extended from \[(4cm\times 2cm)\]to\[(5cm\times 4cm)\]. If the work done is \[3\times {{10}^{-4}}J,\] the value of the surface tension of the liquid is
A)
\[0.250\,N{{m}^{-1}}\]
done
clear
B)
\[0.125\text{ }N{{m}^{-1}}\]
done
clear
C)
\[0.2\text{ }N{{m}^{-1}}\]
done
clear
D)
\[8.0\text{ }N{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 14) Three liquids of densities\[{{\rho }_{1}},{{\rho }_{2}}\]and\[{{\rho }_{3}}\](with \[{{\rho }_{1}}>{{\rho }_{2}}>{{\rho }_{3}})\], having the same value of surface tension T, rise to the same height in three identical capillaries. The angles of contact \[{{\theta }_{1}},{{\theta }_{2}}and\,{{\theta }_{3}}\] obey
A)
\[\frac{\pi }{2}>{{\theta }_{1}}>{{\theta }_{2}}>{{\theta }_{3}}\ge 0\]
done
clear
B)
\[0\le {{\theta }_{1}}<{{\theta }_{2}}<{{\theta }_{3}}<\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{2}\le {{\theta }_{1}}<{{\theta }_{2}}<{{\theta }_{3}}<\pi \]
done
clear
D)
\[\pi >{{\theta }_{1}}>{{\theta }_{2}}>{{\theta }_{3}}>\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 15) One of these is at \[{{100}^{o}}C,\] while the other one is at \[{{10}^{o}}C,\]If the two bodies are brought into contact, then assuming no heat loss, the final common temperature is
A)
\[{{50}^{o}}C\]
done
clear
B)
More than \[{{50}^{o}}C\]
done
clear
C)
Less than\[{{50}^{o}}C\]but greater than \[{{0}^{o}}C\]
done
clear
D)
\[{{0}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 16) A body cools from a temperature 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton's law of cooling is applicable. The temperature of the body at the end of next 10 minutes will be
A)
\[\frac{7}{4}T\]
done
clear
B)
\[\frac{3}{2}T\]
done
clear
C)
\[\frac{4}{3}T\]
done
clear
D)
\[T\]
done
clear
View Answer play_arrow
question_answer 17) One mole of an ideal monatomic gas undergoes a process described by the equation \[P{{V}^{3}}\] constant. The heat capacity of the gas during this process is
A)
\[\frac{3}{2}R\]
done
clear
B)
\[\frac{5}{2}R\]
done
clear
C)
\[2R\]
done
clear
D)
\[R\]
done
clear
View Answer play_arrow
question_answer 18) The temperature inside a refrigerator is \[{{t}_{2}}^{{}^\circ }C\] and the room temperature is \[{{t}_{2}}^{{}^\circ }C.\]The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
A)
\[\frac{{{t}_{1}}}{{{t}_{1}}-{{t}_{2}}}\]
done
clear
B)
\[\frac{{{t}_{1}}+273}{{{r}_{1}}-{{t}_{1}}}\]
done
clear
C)
\[\frac{{{t}_{2}}+273}{{{t}_{1}}-{{t}_{2}}}\]
done
clear
D)
\[\frac{{{t}_{1}}+{{t}_{2}}}{{{t}_{1}}+273}\]
done
clear
View Answer play_arrow
question_answer 19) A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
A)
\[\frac{P}{(KT)}\]
done
clear
B)
\[\frac{Pm}{(KT)}\]
done
clear
C)
\[\frac{P}{(KTV)}\]
done
clear
D)
\[mKT\]
done
clear
View Answer play_arrow
question_answer 20) A body of mass m is attached to the lower end of a spring whose upper end is fixed. The spring has negligible mass. When the mass m is slightly pulled down and released, it oscillates with a time period of 3 s. When the mass m is increased by 1 kg, the time period of oscillations becomes 5 s. The value of m in kg is
A)
\[\frac{3}{4}\]
done
clear
B)
\[\frac{4}{3}\]
done
clear
C)
\[\frac{16}{9}\]
done
clear
D)
\[\frac{9}{16}\]
done
clear
View Answer play_arrow
question_answer 21) The second overtone of an open organ pipe has the same frequency as the first overtone of a closed pipe L metre long. The length of the open pipe will be
A)
L
done
clear
B)
2L
done
clear
C)
\[\frac{L}{2}\]
done
clear
D)
4L
done
clear
View Answer play_arrow
question_answer 22) Three sound waves of equal amplitudes have frequencies\[(n-1),n,(n+1)\].They superimpose to give beats. The number of beats produced per second will be
A)
1
done
clear
B)
4
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 23) An electric dipole is placed at an angle of \[{{30}^{o}}\] with an electric field intensity\[2\times {{10}^{5}}N/C\].It experiences a torque equal to 4 N m. The charge on the dipole, if the dipole length is 2 cm, is
A)
8 mC
done
clear
B)
2 mC
done
clear
C)
5 mC
done
clear
D)
\[2\mu C\]
done
clear
View Answer play_arrow
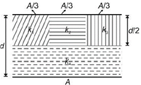
question_answer 24)
A parallel-plate capacitor of area A, plate separation d and capacitance C is filled with four dielectric materials having dielectric constants \[{{K}_{1}},{{K}_{2}},{{K}_{3}}\,and\,{{K}_{4}}\] as shown in the figure below. If a single dielectric material is to be used to have the same capacitance C in this capacitor, then its dielectric constant k is given by
A)
\[K={{K}_{1}}+{{K}_{2}}+{{K}_{3}}+3{{K}_{4}}\]
done
clear
B)
\[K=\frac{2}{3}({{K}_{1}}+{{K}_{2}}+{{K}_{3}})+2{{K}_{4}}\]
done
clear
C)
\[\frac{2}{K}=\frac{3}{{{K}_{1}}+{{K}_{2}}+{{K}_{3}}}+\frac{1}{{{K}_{4}}}\]
done
clear
D)
\[\frac{1}{K}=\frac{1}{{{K}_{1}}}+\frac{1}{{{K}_{2}}}+\frac{1}{{{K}_{3}}}+\frac{3}{2{{K}_{4}}}\]
done
clear
View Answer play_arrow
question_answer 25)
The potential difference \[({{V}_{A}}-{{V}_{B}})\] between the points A and B in the given figure is
A)
-3V
done
clear
B)
+3V
done
clear
C)
+6V
done
clear
D)
+9V
done
clear
View Answer play_arrow
question_answer 26) A filament bulb (500 W, 100 V) is to be used in a 230 V main supply. When a resistance R is connected in series, it works perfectly and the bulb consumes 500 W. The value of R is
A)
\[230\Omega \]
done
clear
B)
\[46\Omega \]
done
clear
C)
\[26\Omega \]
done
clear
D)
\[13\Omega \]
done
clear
View Answer play_arrow
question_answer 27) A long wire carrying a steady current is bent into a circular loop of one turn. The magnetic field at the centre of the loop is B. It is then bent into a circular coil of n turns. The magnetic field at the centre of this coil of n turns will be
A)
nB
done
clear
B)
\[{{n}^{2}}B\]
done
clear
C)
2nB
done
clear
D)
\[2{{n}^{2}}B\]
done
clear
View Answer play_arrow
question_answer 28) A bar magnet is hung by a thin cotton thread in a uniform horizontal magnetic field and is in equilibrium state. The energy required to rotate it by\[{{60}^{o}}\]is W. Now the torque required to keep the magnet in this new position is
A)
\[\frac{W}{\sqrt{3}}\]
done
clear
B)
\[\sqrt{3W}\]
done
clear
C)
\[\frac{\sqrt{3}W}{2}\]
done
clear
D)
\[\frac{2W}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 29) An electron is moving in a circular path under the influence of a transverse magnetic field of\[3.57\times {{10}^{-2}}\] T. If the value of e/m is\[1.67\times {{10}^{11}}C/kg.\] the frequency of revolution of the electron is
A)
1 GHz
done
clear
B)
100 MHz
done
clear
C)
62.8 MHz
done
clear
D)
6.28 MHz
done
clear
View Answer play_arrow
question_answer 30) Which of the following combinations should be selected for better tuning of an L-C-R circuit used for communication?
A)
\[R=20\Omega ,L=1.5\text{ }H,C=35\mu F\]
done
clear
B)
\[R=25\Omega ,L=2.5H,C=45\mu F\]
done
clear
C)
\[R=15\Omega ,L=3.5H,C=30\mu F\]
done
clear
D)
\[R=25\Omega ,L=1.5\text{ }H,C=45\mu F\]
done
clear
View Answer play_arrow
question_answer 31)
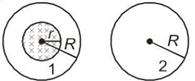
A uniform magnetic field is restricted within a region of radius r. The magnetic field changes with time at a rate\[\frac{d\overset{\to }{\mathop{B}}\,}{dt}\]. Loop 1 of radius \[R>r\] encloses the region r and loop 2 of radius R is outside the region of magnetic field as shown in the figure below. Then the e.m.f. generated is
A)
Zero in loop 1 and zero in loop 2
done
clear
B)
\[-\frac{d\overset{\to }{\mathop{B}}\,}{dt}\pi {{r}^{2}}\text{in loop 1 and}\,-\frac{d\overset{\to }{\mathop{B}}\,}{dt}\pi {{r}^{2}}\,\text{in}\,\text{loop}\,\text{2}\]
done
clear
C)
\[-\frac{d\overset{\to }{\mathop{B}}\,}{dt}\pi {{r}^{2}}\text{in loop 1 and zero in loop 2}\]
done
clear
D)
\[~-\frac{d\overset{\to }{\mathop{B}}\,}{dt}\pi {{r}^{2}}\text{in loop 1 and zero in loop 2}\]
done
clear
View Answer play_arrow
question_answer 32) The potential differences across the resistance, capacitance and inductance are 80 V, 40 V and 100 V respectively in an L-C-R circuit. The power factor of this circuit is
A)
0.4
done
clear
B)
0.5
done
clear
C)
0.8
done
clear
D)
1.0
done
clear
View Answer play_arrow
question_answer 33) A \[100\Omega \] resistance and a capacitor of \[100\Omega \] reactance are connected in series across a 220 V source. When the capacitor is 50% charged, the peak value of the displacement current is
A)
2.2 A
done
clear
B)
11 A
done
clear
C)
4.4 A
done
clear
D)
\[11\sqrt{2A}\]
done
clear
View Answer play_arrow
question_answer 34) Two identical glass \[\left( {{\mu }_{8}}=\frac{3}{2} \right)\] equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water\[\left( {{\mu }_{W}}=\frac{4}{3} \right)\]. The focal length of the combination is
A)
\[\frac{f}{3}\]
done
clear
B)
f
done
clear
C)
\[\frac{4f}{3}\]
done
clear
D)
\[\frac{3f}{4}\]
done
clear
View Answer play_arrow
question_answer 35) An air bubble in a glass slab with refractive index 1.5 (near normal incidence) is 5 cm deep when viewed from one surface and 3 cm deep when viewed from the opposite face. The thickness (in cm) of the slab is
A)
8
done
clear
B)
10
done
clear
C)
12
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 36) The interference pattern is obtained with two coherent light sources of intensity ratio n. In the interference pattern, the ratio \[\frac{{{\operatorname{I}}_{\max }}-{{\operatorname{I}}_{\min }}}{{{I}_{\max }}+{{I}_{\min }}}\]will be
A)
\[\frac{\sqrt{n}}{n+1}\]
done
clear
B)
\[\frac{2\sqrt{n}}{n+1}\]
done
clear
C)
\[\frac{\sqrt{n}}{{{(n+1)}^{2}}}\]
done
clear
D)
\[\frac{2\sqrt{n}}{{{(n+1)}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 37) A person can see clearly objects only when they lie between 50 cm and 400 cm from his eyes. In order to increase the maximum distance of distinct vision to infinity, the type and power of the correcting lens, the person has to use, will be
A)
Convex, +2.25 diopter
done
clear
B)
Concave, \[-0.25\]diopter
done
clear
C)
Concave, \[-0.2\]diopter
done
clear
D)
Convex, + 0.15 diopter
done
clear
View Answer play_arrow
question_answer 38) A linear aperture whose width is 0.02 cm is placed immediately in front of a lens of focal length 60 cm. The aperture is illuminated normally by a parallel beam of wavelength\[5\times {{10}^{-5}}cm\].The distance of the first dark band of the diffraction pattern from the centre of the screen is
A)
0.10 cm
done
clear
B)
0.25 cm
done
clear
C)
0.20 cm
done
clear
D)
0.15 cm
done
clear
View Answer play_arrow
question_answer 39) Electrons of mass m with de-Broglie wavelength λ fall on the target in an X-ray tube. The cutoff wavelength \[{{\lambda }_{0}}\] of the emitted X-ray is
A)
\[{{\lambda }_{0}}=\frac{2mc{{\lambda }^{2}}}{h}\]
done
clear
B)
\[{{\lambda }_{0}}=\frac{2h}{mc}\]
done
clear
C)
\[{{\lambda }_{0}}=\frac{2{{m}^{2}}{{c}^{2}}{{\lambda }^{2}}}{{{h}^{2}}}\]
done
clear
D)
\[{{\lambda }_{0}}=\lambda \]
done
clear
View Answer play_arrow
question_answer 40) Photons with energy 5 eV are incident on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative to C is
A)
+3 V
done
clear
B)
+4 V
done
clear
C)
-1 V
done
clear
D)
-3 V
done
clear
View Answer play_arrow
question_answer 41) If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd orbit, it emits a photon of wavelength λ. When it jumps from the 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be
A)
\[\frac{16}{25}\lambda \]
done
clear
B)
\[\frac{9}{16}\lambda \]
done
clear
C)
\[\frac{20}{7}\lambda \]
done
clear
D)
\[\frac{20}{13}\lambda \]
done
clear
View Answer play_arrow
question_answer 42) The half-life of a radioactive substance is 30 minutes. The time (in minutes) taken between 40% decay and 85% decay of the same radioactive substance is
A)
15
done
clear
B)
30
done
clear
C)
45
done
clear
D)
60
done
clear
View Answer play_arrow
question_answer 43) For CE transistor amplifier, the audio signal voltage across the collector resistance of 2 kΩ is 4 V. If the current amplification factor of the transistor is 100 and the base resistance is 1 k Ω then the input signal voltage is
A)
10 mV
done
clear
B)
20 mV
done
clear
C)
30 mV
done
clear
D)
15 mV
done
clear
View Answer play_arrow
question_answer 44) The given circuit has two ideal diodes connected as shown in the figure below. The current flowing through the resistance \[{{R}_{1}}\]will be
A)
2.5 A
done
clear
B)
10.0 A
done
clear
C)
1.43 A
done
clear
D)
3.13 A
done
clear
View Answer play_arrow
question_answer 45)
What is the output Y in the following circuit, when all the three inputs A, B, C are first 0 and then 1?
A)
0, 1
done
clear
B)
0, 0
done
clear
C)
1, 0
done
clear
D)
1, 1
done
clear
View Answer play_arrow
question_answer 46) Which one of the following compounds shows the presence of intermolecular hydrogen bond?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
HCN
done
clear
C)
Cellulose
done
clear
D)
Concentrated acetic acid
done
clear
View Answer play_arrow
question_answer 47) The molar conductivity of a\[0.5\frac{mol}{d{{m}^{3}}}\]solution of \[AgN{{O}_{3}}\]with electrolytic conductivity of \[5.76\times {{10}^{-3}}S\text{ }c{{m}^{-1}}at\text{ }298K\]
A)
\[2.88\text{ }Sc{{m}^{2}}/mol\]
done
clear
B)
\[11.52\text{ }Sc{{m}^{2}}/mol\]
done
clear
C)
\[0.086\text{ }Sc{{m}^{2}}/mol\]
done
clear
D)
\[28.8\text{ }Sc{{m}^{2}}/mol\]
done
clear
View Answer play_arrow
question_answer 48) The decomposition of phosphine\[(P{{H}_{3}})\]on tungsten at low pressure is a first-order reaction. It is because the
A)
Rate is proportion to the surface coverage
done
clear
B)
Rate is inversely proportional to the surface coverage
done
clear
C)
Rate is independent of the surface coverage
done
clear
D)
Rate of decomposition is very slow
done
clear
View Answer play_arrow
question_answer 49)
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of \[A{{s}_{2}}{{S}_{3}}\] are given below: I. \[(NaCl)=52\] I. \[(BaC{{l}_{2}})=0.69\] III. \[(MgS{{o}_{4}})=0.22\]
The <b>correct</b> order of their coagulating power is
A)
I>II>III
done
clear
B)
II>I>III
done
clear
C)
III>II>I
done
clear
D)
III>I>II
done
clear
View Answer play_arrow
question_answer 50) During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
A)
55 minutes
done
clear
B)
110 minutes
done
clear
C)
220 minutes
done
clear
D)
330 minutes
done
clear
View Answer play_arrow
question_answer 51) How many electrons can fit in the orbital for which \[~n=3and\text{ }l=1?\]
A)
2
done
clear
B)
6
done
clear
C)
10
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 52) For a sample of perfect gas when its pressure is changed isothermally from \[{{p}_{1}}to\text{ }{{p}_{2}}\], the entropy change is given by
A)
\[\Delta S=nrIn\left( \frac{Pf}{Pi} \right)\]
done
clear
B)
\[\Delta S=nrIn\left( \frac{Pi}{Pf} \right)\]
done
clear
C)
\[\Delta S=nrTIn\left( \frac{Pf}{Pi} \right)\]
done
clear
D)
\[\Delta S=RTIn\left( \frac{Pi}{Pf} \right)\]
done
clear
View Answer play_arrow
question_answer 53) The van't Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 54) The percentage of pyridine \[({{C}_{5}}{{H}_{5}}N)\] that forms pyridinium ion \[({{C}_{5}}{{H}_{5}}{{N}^{+}}H)\] in a 0.10 M aqueous pyridine solution \[({{K}_{b}}\,for\text{ }{{C}_{5}}{{H}_{5}}N=1.7\times {{10}^{-9}}\,\]is
A)
0.0060%
done
clear
B)
0.013%
done
clear
C)
0.77%
done
clear
D)
1.6%
done
clear
View Answer play_arrow
question_answer 55) In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion \[(C{{a}^{2+}})\] and fluoride ion\[({{F}^{-}})\]are
A)
4 and 2
done
clear
B)
6 and 6
done
clear
C)
8 and 4
done
clear
D)
4 and 8
done
clear
View Answer play_arrow
question_answer 56) If the \[{{E}^{{}^\circ }}_{cell}\]for a given reaction has a negative value, which of the following gives the <b>correct</b> relationships for the values of \[\Delta {{G}^{0}}\]and\[{{K}_{eq}}\]?
A)
\[\Delta {{G}^{o}}\text{0; }{{K}_{eq}}<1\]
done
clear
B)
\[\Delta {{G}^{o}}>0;{{K}_{eq}}>1\]
done
clear
C)
\[\Delta {{G}^{o}}<0;{{K}_{eq}}<1\]
done
clear
D)
\[\Delta {{G}^{o}}<0;{{K}_{eq}}<1\]
done
clear
View Answer play_arrow
question_answer 57) Which one of the following is <b>incorrect</b> for ideal solution?
A)
\[\Delta {{H}_{mix}}=0\]
done
clear
B)
\[\Delta {{U}_{mix}}=0\]
done
clear
C)
\[\Delta P={{0}_{\text{obs}}}-{{P}_{\text{calculated}\,\text{by}\,\text{Raiul }\!\!\grave{\ }\!\!\text{ s}\,\text{law}}}=0\]
done
clear
D)
\[\Delta {{G}_{mix}}\,for\,ideal\,Solution\,is\,positive\]
done
clear
View Answer play_arrow
question_answer 58) The solubility of \[AgCl(s)\] with solubility product \[1.6\times {{10}^{-10}}\]in 0.1 M \[NaCl\] solution would be
A)
\[1.26\times {{10}^{-5}}M\]
done
clear
B)
\[1.6\times {{10}^{-11}}M\]
done
clear
C)
\[1.6\times {{10}^{-9}}M\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 59) Suppose the elements X and Y combine to form two compounds\[X{{Y}_{2}}\]and \[{{X}_{3}}{{Y}_{2}}\]. When 0.1 mole of\[X{{Y}_{2}}\]weighs 10 g and 0.05 mole of\[{{X}_{3}}{{Y}_{2}}\] weighs 9 g, the atomic weights of X and Y are
A)
40, 30
done
clear
B)
60, 40
done
clear
C)
20, 30
done
clear
D)
30, 20
done
clear
View Answer play_arrow
question_answer 60) The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron \[=1.60\times {{10}^{-19}}C)\]
A)
\[6\times {{10}^{23}}\]
done
clear
B)
\[6\times {{10}^{20}}\]
done
clear
C)
\[3.75\times {{10}^{20}}\]
done
clear
D)
\[7.48\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 61) Boric acid is an acid because its molecule
A)
Contains replaceable \[{{H}^{+}}\]ion
done
clear
B)
Gives up a proton
done
clear
C)
Accepts\[O{{H}^{-}}\]from water releasing proton
done
clear
D)
Combines with proton from water molecule
done
clear
View Answer play_arrow
question_answer 62) \[Al{{F}_{3}}\]is soluble in HF only in presence of KF. It is due to the formation of
A)
\[{{K}_{2}}[Al{{F}_{3}}{{H}_{3}}]\]
done
clear
B)
\[{{K}_{3}}[Al{{F}_{6}}]\]
done
clear
C)
\[Al{{H}_{3}}\]
done
clear
D)
\[K(A{{l}_{3}}H)\]
done
clear
View Answer play_arrow
question_answer 63) Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
A)
Zinc is lighter than iron
done
clear
B)
Zinc has lower melting point than iron
done
clear
C)
Zinc has lower negative electrode potential than iron
done
clear
D)
Zinc has higher negative electrode potential than iron
done
clear
View Answer play_arrow
question_answer 64) The suspension of slaked lime in water is known as
A)
Limewater
done
clear
B)
Quicklime
done
clear
C)
Milk of lime
done
clear
D)
Aqueous solution of slaked lime
done
clear
View Answer play_arrow
question_answer 65) The hybridizations of atomic orbitals of nitrogen in\[NO_{2}^{+},NO_{3}^{-}\]and\[N{{H}^{+}}_{4}\]respectively are
A)
\[sp,\,\,s{{p}^{2}}and\,s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{2}},\,\,s{{p}^{3}}and\,s{{p}^{{}}}\]
done
clear
C)
\[s{{p}^{{}}},\,\,s{{p}^{2}}and\,s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{2}},\,\,s{{p}^{{}}}and\,s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following fluoro - compounds is most likely to behave as a Lewis base?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[P{{F}_{3}}\]
done
clear
C)
\[C{{F}_{4}}\]
done
clear
D)
\[S{{i}_{4}}\]
done
clear
View Answer play_arrow
question_answer 67) Which of the following pairs of ions is isoelectronic and is structural?
A)
\[Co_{3}^{2-},NO_{3}^{-}\]
done
clear
B)
\[Cl_{3}^{-},CO_{3}^{2-}\]
done
clear
C)
\[SO_{3}^{2-},NO_{3}^{-}\]
done
clear
D)
\[ClO_{2}^{-},SO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 68) In context with beryllium which one of the following statements is <b>incorrect</b>
A)
It is rendered passive by nitric acid
done
clear
B)
It form \[B{{e}_{2}}C\]
done
clear
C)
Its salts rarely hydrolyze
done
clear
D)
Its hydride is electron -deficient and polymeric
done
clear
View Answer play_arrow
question_answer 69) Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour?
A)
\[C{{u}^{+}}2{{H}_{2}}S{{O}_{4}}\to CuS{{O}_{4}}+S{{O}_{2}}+2{{H}_{2}}O\]
done
clear
B)
\[3S+2{{H}_{2}}S{{O}_{4}}\to 3S{{O}_{2}}+2{{H}_{2}}O\]
done
clear
C)
\[C+2{{H}_{2}}S{{O}_{4}}\to C{{O}_{2}}+2S{{O}_{2}}+2{{H}_{2}}O\]
done
clear
D)
\[C{{a}_{2}}+{{H}_{2}}S{{O}_{4}}\to CaS{{O}_{4}}+2HF\]
done
clear
View Answer play_arrow
question_answer 70) Which of the following pairs of d - orbitals will have electron density along the axes?
A)
\[{{d}_{{{z}^{2}}}},{{d}_{xz}}\]
done
clear
B)
\[{{d}_{xz}},{{d}_{yz}}\]
done
clear
C)
\[{{d}_{{{z}^{2}}}},{{d}_{{{x}^{2}}}}-{{y}^{2}}\]
done
clear
D)
\[{{d}_{xy}},{{d}_{{{x}^{2}}}}-{{y}^{2}}\]
done
clear
View Answer play_arrow
question_answer 71) The correct geometry and hybridization for\[Xe{{F}_{4}}\] are
A)
Octahedral, \[s{{p}^{3}}{{d}^{2}}\]
done
clear
B)
Trigonal bipyramidal, \[s{{p}^{3}}d\]
done
clear
C)
Planar triangle, \[s{{p}^{3}}{{d}^{3}}\]
done
clear
D)
Square planar, \[s{{p}^{3}}{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 72) Among the following, which one is a wrong statement?
A)
\[P{{H}_{5}}\,and\,BiC{{l}_{5}}\,\] do not exist
done
clear
B)
\[p\pi -d\pi \]bonds are present in \[S{{O}_{4}}\]
done
clear
C)
\[SeF{{O}_{4}}\] and\[C{{H}_{4}}\]have same shape
done
clear
D)
\[I_{3}^{+}\] has bent geometry
done
clear
View Answer play_arrow
question_answer 73) The correct increasing order of trans - effect of the following species is
A)
\[N{{H}_{3}}>C{{N}^{-}}>B{{r}^{-}}>{{C}_{6}}H_{5}^{-}\]
done
clear
B)
\[C{{N}^{-}}>{{C}_{6}}H_{5}^{-}>B{{r}^{-}}>N{{H}_{3}}\]
done
clear
C)
\[B{{r}^{-}}>C{{N}^{-}}>N{{H}_{3}}>{{C}_{6}}H_{5}^{-}\]
done
clear
D)
\[C{{N}^{-}}>B{{r}^{-}}>{{C}_{6}}H_{5}^{-}>N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 74) Which one of the following statements related to lanthanons is incorrect?
A)
Europium shows + 2 oxidation state
done
clear
B)
The basicity decreases as the ionic radius decreases from Pr to Lu
done
clear
C)
All the lanthanons are much more reactive than aluminium
done
clear
D)
Ce (+4) solutions are widely used as oxidizing agent in volumetric analysis
done
clear
View Answer play_arrow
question_answer 75) Jahn - Teller effect is not observed in high spin complexes of
A)
\[{{d}^{7}}\]
done
clear
B)
\[{{d}^{8}}\]
done
clear
C)
\[{{d}^{4}}\]
done
clear
D)
\[{{d}^{9}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following can be used as the halide component for Friedel Crafts reaction?
A)
Chlorobenzene
done
clear
B)
Bromobenzene
done
clear
C)
Chloroethene
done
clear
D)
Isopropyl chloride
done
clear
View Answer play_arrow
question_answer 77) In which of the following molecules, all atoms are coplanar?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 78) Which one of the following structures represents nylon 6,6 polymer ?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 79)
In pyrrole the electron density s maximum on
A)
2 and 3
done
clear
B)
3 and 4
done
clear
C)
2 and 4
done
clear
D)
2 and 5
done
clear
View Answer play_arrow
question_answer 80) Which of the following compounds shall not produce propene by reaction with HBr followed by elimination or direct only elimination reaction?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 81) Which one of the following nitro - compounds does not react with nitrous acid?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 82) The central dogma of molecular genetics states that the genetic information flows from
A)
Amino acids \[\to \] Proteins \[\to \] DNA
done
clear
B)
DNA \[\to \] Carbohydrates \[\to \] Proteins
done
clear
C)
DNA \[\to \] RNA \[\to \] Proteins
done
clear
D)
DNA \[\to \] RNA \[\to \] Carbohydrates
done
clear
View Answer play_arrow
question_answer 83)
The correct corresponding order of names of four aldoses with configuration given below respectively, is
A)
L - erythrose, L - threose, L - erythrose, D - threose
done
clear
B)
D - threose, D - erythrose, L - threose, L - erythrose
done
clear
C)
L - erythrose, L - threose, D - erythrose, D - threose
done
clear
D)
D - erythrose, D - threose, L - erythrose, L - threose
done
clear
View Answer play_arrow
question_answer 84)
In the given reaction the product P is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85) A given nitrogen - containing aromatic compound A reacts with Sn⁄HCl, followed by \[HN{{O}_{3}}\]to give an unstable compound B.B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula \[{{C}_{12}}{{H}_{10}}{{N}_{2}}O\]. The structure of compound A is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 86) Consider the reaction\[C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Br+Na\to C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}CN+NaBr\]. This reaction will be the fastest in
A)
Ethanol
done
clear
B)
Methanol
done
clear
C)
N, N? - dimethylformamde (DMF)
done
clear
D)
Water
done
clear
View Answer play_arrow
question_answer 87)
The correct structure of the product A formed in the reaction is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 88)
Which among the given molecules can exhibit tautomerism?
A)
III Only
done
clear
B)
Both I and III
done
clear
C)
Both I and II
done
clear
D)
Both II and III
done
clear
View Answer play_arrow
question_answer 89)
The correct order of strengths of the carboxylic acids is
A)
I > II > III
done
clear
B)
II > III > I
done
clear
C)
III > II > I
done
clear
D)
II > I > III
done
clear
View Answer play_arrow
question_answer 90) The compound that will react most readily with gaseous bromine has the formula
A)
\[{{C}_{3}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
C)
\[{{C}_{4}}{{H}_{10}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) Which one of the following is wrong for fungi?
A)
They are eukaryotic
done
clear
B)
All fungi possess a purely cellulosic cell wall
done
clear
C)
They are heterotrophic
done
clear
D)
They are both unicellular and multicellular
done
clear
View Answer play_arrow
question_answer 92) Methanogens belong to
A)
Eubacteria
done
clear
B)
Archaebacteria
done
clear
C)
Dinoflagellates
done
clear
D)
Slime moulds
done
clear
View Answer play_arrow
question_answer 93) Select the wrong statement.
A)
The walls of diatoms are easily destructible
done
clear
B)
"Diatomaceous earth" is formed by the cell walls of diatoms
done
clear
C)
Diatoms are chief producers in the oceans
done
clear
D)
Diatoms are microscopic and float passively in water
done
clear
View Answer play_arrow
question_answer 94) The label of a herbarium sheet does not carry information on
A)
Data of collection
done
clear
B)
Name of collector
done
clear
C)
Local names
done
clear
D)
Height of the plant
done
clear
View Answer play_arrow
question_answer 95) Conifers are adapted to tolerate extreme environmental conditions because of
A)
Broad hardy leaves
done
clear
B)
Superficial stomata
done
clear
C)
Thick cuticle
done
clear
D)
Presence of vessels
done
clear
View Answer play_arrow
question_answer 96) Which one of the following statements is wrong?
A)
Algae increase the level of dissolved oxygen in the immediate environment
done
clear
B)
Algin is obtained from red algae, and carrageenan from brown algae
done
clear
C)
Agar - agar is obtained from Gelidium and Gracilaria
done
clear
D)
Laminaria and Sargassum are used as food
done
clear
View Answer play_arrow
question_answer 97) The term "polyadelphous" is related to
A)
Gynoecium
done
clear
B)
Androecium
done
clear
C)
Corolla
done
clear
D)
Calyx
done
clear
View Answer play_arrow
question_answer 98) How many plants among Indigofera, Sesbania, Salvia, Allium, Aloe, mustard, groundnut, radish, gram and turnip have stamens with different lengths in their flowers?
A)
Three
done
clear
B)
Four
done
clear
C)
Five
done
clear
D)
Six
done
clear
View Answer play_arrow
question_answer 99) Radial symmetry is found in the flowers of
A)
Brassica
done
clear
B)
Trifolium
done
clear
C)
Pisum
done
clear
D)
Cassia
done
clear
View Answer play_arrow
question_answer 100) Free-central placentation is found in
A)
Dianthus
done
clear
B)
Argemone
done
clear
C)
Brassica
done
clear
D)
Citrus
done
clear
View Answer play_arrow
question_answer 101) Cortex is the region found between
A)
Epidermis and stele
done
clear
B)
Pericycle and endodermis
done
clear
C)
Endodermis and pith
done
clear
D)
Endodermis and vascular bundle
done
clear
View Answer play_arrow
question_answer 102) The balloon - shaped structures called tyioses
A)
Originate in the lumen of vessels
done
clear
B)
Characterize the sapwood
done
clear
C)
Are extensions of xylem parenchyma cells into vessels
done
clear
D)
Are linked to the ascent of sap through xylem vessels
done
clear
View Answer play_arrow
question_answer 103) A non - proteinaceous enzyme is
A)
Lysozyme
done
clear
B)
Ribozyme
done
clear
C)
Ligase
done
clear
D)
Deoxyribonuclease
done
clear
View Answer play_arrow
question_answer 104) Select the mismatch.
A)
Gas vacuoles - Green bacteria
done
clear
B)
Large central vacuoles - Animal cells
done
clear
C)
Protists - Eukaryotes
done
clear
D)
Methanogens - Prokaryotes
done
clear
View Answer play_arrow
question_answer 105) Select the wrong statement
A)
Bacteria cell wall is made up of peptidoglycan
done
clear
B)
Pili and fimbriae are mainly involved in motility of bacterial cells
done
clear
C)
Cyanobacteria lack flagellated cells
done
clear
D)
Mycoplasma is a wall - less microorganism
done
clear
View Answer play_arrow
question_answer 106) A cell organelle containing hydrolytic enzymes is
A)
Lysosome
done
clear
B)
Microsome
done
clear
C)
Ribosome
done
clear
D)
Mesosome
done
clear
View Answer play_arrow
question_answer 107) During cell growth, DNA synthesis takes place in
A)
S phase
done
clear
B)
\[{{G}_{1}}\]phase
done
clear
C)
\[{{G}_{2}}\]phase
done
clear
D)
M phase
done
clear
View Answer play_arrow
question_answer 108) Which of the following biomolecules is common to respiration - mediated breakdown of fats, carbohydrates and proteins?
A)
Glucose - 6 - phosphate
done
clear
B)
Fructose 1, 6- bisphosphate
done
clear
C)
Pyruvic acid
done
clear
D)
Acetyl CoA
done
clear
View Answer play_arrow
question_answer 109) A few drops of sap were collected by cutting across a plant stem by a suitable method. The sap was tested chemically. Which one of the following test results indicates that it is phloem sap?
A)
Acidic
done
clear
B)
Alkaline
done
clear
C)
Low refractive index
done
clear
D)
Absence of sugar
done
clear
View Answer play_arrow
question_answer 110) You are given a tissue with its potential for differentiation in an artificial culture. Which of the following pairs of hormones would you add to the medium to secure shoots as well as roots?
A)
IAA and gibberellin
done
clear
B)
Auxin and cytokinin
done
clear
C)
Auxin and abscisic acid
done
clear
D)
Gibberellin and abscisic acid
done
clear
View Answer play_arrow
question_answer 111) Phytochrome is a
A)
Flavoprotein
done
clear
B)
Glycoprotein
done
clear
C)
Lipoprotein
done
clear
D)
Chromoprotein
done
clear
View Answer play_arrow
question_answer 112) Which is essential for the growth of root tip?
A)
\[Zn\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Ca\]
done
clear
D)
\[Mn\]
done
clear
View Answer play_arrow
question_answer 113) The process which makes major difference between \[{{C}_{3}}\] and\[{{C}_{4}}\]. plants is
A)
Glycolysis
done
clear
B)
Calvin cycle
done
clear
C)
Photorespiration
done
clear
D)
Respiration
done
clear
View Answer play_arrow
question_answer 114) Which one of the following statements is not correct?
A)
Offspring produced by the asexual reproduction are called clone
done
clear
B)
Microscopic, motile asexual reproductive structures are called zoospores
done
clear
C)
In potato, banana and ginger, the plantlets arise from the internodes present in the modified stem
done
clear
D)
Water hyacinth, growing in the standing water, drains oxygen from water that leads to the death of fishes
done
clear
View Answer play_arrow
question_answer 115) Which one of the following generates new genetic combinations leading to variation?
A)
Vegetative reproduction
done
clear
B)
Parthenogenesis
done
clear
C)
Sexual reproduction
done
clear
D)
Nucellar polyembryony
done
clear
View Answer play_arrow
question_answer 116)
Match Column - I with Column - II and select the correct option using the codes given below.
Column - I
Column - II
A. Pistils fused together
(i) Gametogenesis
B. Formation of gametes
(ii) Pistillate
C. Hyphae of higher
(iii) Syncarpous
D. Unisexual female flower
(iv) Dikaryotic
A)
\[\text{A-iv B-iii C-i D-ii}\]
done
clear
B)
\[\text{A-ii B-i C-iv D-iii}\]
done
clear
C)
\[\text{A-i B-ii C-iv D-iii}\]
done
clear
D)
\[\text{A-iii B-i C-iv D-ii}\]
done
clear
View Answer play_arrow
question_answer 117) In majority of angiosperms
A)
Egg has a filiform apparatus
done
clear
B)
There are numerous antipodal cells
done
clear
C)
Reduction division occurs in the megaspore
done
clear
D)
A small central cell is present in the embryo sac
done
clear
View Answer play_arrow
question_answer 118) Polllination in water hyacinth and water lily is brough about by the agency of
A)
Water
done
clear
B)
Insects or wind
done
clear
C)
Birds
done
clear
D)
Bats
done
clear
View Answer play_arrow
question_answer 119) The ovule of an angiosperm is technically equivalent to
A)
Megasporangium
done
clear
B)
Megasporophyll
done
clear
C)
Megaspore mother cell
done
clear
D)
Megaspore
done
clear
View Answer play_arrow
question_answer 120) Taylor conducted the experiments to prove semiconservative mode of chromosome replication on
A)
Vinca rosea
done
clear
B)
Viciafaba
done
clear
C)
Drosophila melanogaster
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 121) The mechanism that causes a gene to move from one linkage group to another is called
A)
Inversion
done
clear
B)
Duplication
done
clear
C)
Translocation
done
clear
D)
Crossing-over
done
clear
View Answer play_arrow
question_answer 122) The equivalent of a structural gene is
A)
Muton
done
clear
B)
Cistron
done
clear
C)
Operon
done
clear
D)
Recon
done
clear
View Answer play_arrow
question_answer 123) A true breeding plant is
A)
One that is able to breed on its own
done
clear
B)
Produced due to cross-pollination among unrelated plants
done
clear
C)
Near homozygous and produces offspring of its own kind
done
clear
D)
Always homozygous recessive in its genetic constitution
done
clear
View Answer play_arrow
question_answer 124) Which of the following rRNAs acts as structural RNA as well as ribozyme in bacteria?
A)
5 S rRNA
done
clear
B)
18 S rRNA
done
clear
C)
23 S rRNA
done
clear
D)
5.8 S rRNA
done
clear
View Answer play_arrow
question_answer 125) Stirred-tank bioreactors have been designed for
A)
Purification of product
done
clear
B)
Addition of preservatives to the product
done
clear
C)
Availability of oxygen throughout the process
done
clear
D)
Ensuring anaerobic conditions in the culture vessel
done
clear
View Answer play_arrow
question_answer 126) A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using
A)
Eco Rl
done
clear
B)
Taq polymerase
done
clear
C)
Polymerase III
done
clear
D)
Ligase
done
clear
View Answer play_arrow
question_answer 127) Which of the following is not a component of downstream processing?
A)
Separation
done
clear
B)
Purification
done
clear
C)
Preservation
done
clear
D)
Expression
done
clear
View Answer play_arrow
question_answer 128) Which of the following restriction enzymes produces blunt ends?
A)
Sal I
done
clear
B)
Eco RV
done
clear
C)
Xhol
done
clear
D)
Hind III
done
clear
View Answer play_arrow
question_answer 129) Which kind of therapy was given in 1990 to a four year- old girl with adenosine deaminase (ADA) deficiency?
A)
Gene therapy
done
clear
B)
Chemotherapy
done
clear
C)
Immunotherapy
done
clear
D)
Radiation therapy
done
clear
View Answer play_arrow
question_answer 130) How many hot spots of biodiversity in the world have been identified till date by Norman Myers?
A)
17
done
clear
B)
25
done
clear
C)
34
done
clear
D)
43
done
clear
View Answer play_arrow
question_answer 131) The primary producers of the deep-sea hydrothermal vent ecosystem are
A)
Green algae
done
clear
B)
Chemosynthetic bacteria
done
clear
C)
Blue-green algae
done
clear
D)
Coral reefs
done
clear
View Answer play_arrow
question_answer 132) Which of the following is correct for r-selected species?
A)
Large number of progeny with small size
done
clear
B)
Large number of progeny with large size
done
clear
C)
Small number of progeny with small size
done
clear
D)
Small number of progeny with large size
done
clear
View Answer play_arrow
question_answer 133) If '+' sign is assigned to beneficial interaction,'-' sign to detrimental and '0' sign to neutral interaction, then the population interaction represented by '+', '-' refers to
A)
Mutualism
done
clear
B)
Amensalism
done
clear
C)
Commensalism
done
clear
D)
Parasitism
done
clear
View Answer play_arrow
question_answer 134) Which of the following is correctly matched?
A)
Aerenchyma - Opuntia
done
clear
B)
Age pyramid - Biome
done
clear
C)
Parthenium hysterophorus - Threat to Biodiversity
done
clear
D)
Stratification - Population
done
clear
View Answer play_arrow
question_answer 135) Red list contains data or information on
A)
All economically important plants
done
clear
B)
Plants whose products are in international trade
done
clear
C)
Threatened species
done
clear
D)
Marine vertebrates only
done
clear
View Answer play_arrow
question_answer 136) Which of the following sets of diseases is caused by bacteria?
A)
Cholera and tetanus
done
clear
B)
Typhoid and smallpox
done
clear
C)
Tetanus and mumps
done
clear
D)
Herpes and influenza
done
clear
View Answer play_arrow
question_answer 137)
Match Column-I with Column-II for housefly classification and select the correct option using the codes given below:
Column - I
Column - II
A. Family
(i) Diplera
B. Order
(ii) Arthropoda
C. Class
(iii) Muscidae
D. Phylum
(iv) Insecta
A)
A - (iii) B - (i) C - (iv) D - (ii)
done
clear
B)
A - (iii) B - (ii) C - (iv) D - (i)
done
clear
C)
A - (iv) B - (iii) C - (ii) D - (i)
done
clear
D)
A - (iv) B - (ii) C - (i) D - (iii)
done
clear
View Answer play_arrow
question_answer 138) Choose the correct statement.
A)
All mammals are viviparous
done
clear
B)
All cyclostomes do not possess jaws and paired fins.
done
clear
C)
All reptiles have a three-chambered heart.
done
clear
D)
All pisces have gills covered by an operculum.
done
clear
View Answer play_arrow
question_answer 139)
Study the four statements (A- D) given below and select the two correct ones out of them: A. Definition of biological species was given by Ernst Mayr. B. Photoperiod does not affect reproduction in plants. C. Binomial nomenclature system was given by R.H. Whittaker. D. In unicellular organisms, reproduction is synonymous with growth.
The two correct statements are
A)
B and C
done
clear
B)
C and D
done
clear
C)
A and D
done
clear
D)
A and B
done
clear
View Answer play_arrow
question_answer 140) In male cockroaches, sperms are stored in which part of the reproductive system?
A)
Seminal vesicles
done
clear
B)
Mushroom glands
done
clear
C)
Testes
done
clear
D)
Vas deferens
done
clear
View Answer play_arrow
question_answer 141) Smooth muscles are
A)
Involuntary, fusiform, non-striated
done
clear
B)
Voluntary, multinucleate, cylindrical
done
clear
C)
Involuntary, cylindrical, striated
done
clear
D)
Voluntary, spindle-shaped, uninucleate
done
clear
View Answer play_arrow
question_answer 142) Oxidative phosphorylation is
A)
Formation of ATP by transfer of phosphate group from a substrate to ADP
done
clear
B)
Oxidation of phosphate group in ATP
done
clear
C)
Addition of phosphate group to ATP
done
clear
D)
Formation of ATP by energy released from electrons removed during substrate oxidation
done
clear
View Answer play_arrow
question_answer 143) Which of the following is the least likely to be involved in stabilizing the three-dimensional folding of most proteins?
A)
Hydrogen bonds
done
clear
B)
Electrostatic interaction
done
clear
C)
Hydrophobic interaction
done
clear
D)
Ester bonds
done
clear
View Answer play_arrow
question_answer 144)
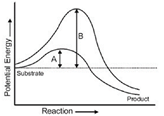
Which of the following describes the given graph correctly?
A)
Endothermic reaction with energy A in presence of enzyme and B in absence of enzyme
done
clear
B)
Exothermic reaction with energy A in presence of enzyme and B in absence of enzyme
done
clear
C)
Endothermic reaction with energy A in absence of enzyme and B in presence of enzyme
done
clear
D)
Exothermic reaction with energy A in absence of enzyme and B in presence of enzyme
done
clear
View Answer play_arrow
question_answer 145) When cell has stalled DNA replication fork, which checkpoint should be predominantly activated?
A)
\[{{G}_{1}}/S\]
done
clear
B)
\[{{G}_{2}}/M\]
done
clear
C)
M
done
clear
D)
Both \[{{G}_{2}}/M\] and \[M\]
done
clear
View Answer play_arrow
question_answer 146)
Match the stages of meiosis of Column-I to their characteristic features in Column-II and select the correct option using the codes given below: Column - I Column - II a. Pachytene (i) Pairing of homologous chromosomes b. Metaphasel (ii) Terminalization of chiasmata c. Diainesis (iii) Crossing - over takes place d. Zygotene (iv) Chromosomes align at equatorial plate
A)
A - (iii) B - (iv) C - (ii) D - (i)
done
clear
B)
A - (i) B - (iv) C - (ii) D - (iii)
done
clear
C)
A - (ii) B - (iv) C - (iii) D - (i)
done
clear
D)
A - (iv) B - (iii) C - (ii) D - (i)
done
clear
View Answer play_arrow
question_answer 147) Which hormones do stimulate the production of pancreatic juice and bicarbonate?
A)
Angiotensin and epinephrine
done
clear
B)
Gastrin and insulin
done
clear
C)
Cholecystokinin and secretin
done
clear
D)
Insulin and glucagon
done
clear
View Answer play_arrow
question_answer 148) The partial pressure of oxygen in the alveoli of the lungs is
A)
Equal to that in the blood
done
clear
B)
More than that in the blood
done
clear
C)
Less than that in the blood
done
clear
D)
Less than that of carbon dioxide
done
clear
View Answer play_arrow
question_answer 149) Choose the correct statements.
A)
Nociceptors respond to changes in pressure
done
clear
B)
Meissner's corpuscles are thermoreceptors
done
clear
C)
Photoreceptors in the human eye are depolarised during darkness and become hyperpolarized in response to the light stimulus
done
clear
D)
Receptors do not produce graded potentials
done
clear
View Answer play_arrow
question_answer 150) Graves' disease is caused due to
A)
Hyposecretion of thyroid gland
done
clear
B)
Hypersecretion of thyroid gland
done
clear
C)
Hyposecretion of adrenal gland
done
clear
D)
Hypersecretion of adrenal gland
done
clear
View Answer play_arrow
question_answer 151) Name the ion responsible for unmasking of active sites for myosin for cross-bridge activity during muscle contraction.
A)
Calcium
done
clear
B)
Magnesium
done
clear
C)
Sodium
done
clear
D)
Potassium
done
clear
View Answer play_arrow
question_answer 152) Name the blood cells, whose reduction in number can cause clotting disorder, leading to excessive loss of blood from the body.
A)
Erythrocytes
done
clear
B)
Leucocytes
done
clear
C)
Neutrophils
done
clear
D)
Thrombocytes
done
clear
View Answer play_arrow
question_answer 153) Name a peptide hormone which acts mainly on hepatocytes, adipocytes and enhances cellular glucose uptake and utilization.
A)
Insulin
done
clear
B)
Glucagon
done
clear
C)
Secretin
done
clear
D)
Gastrin
done
clear
View Answer play_arrow
question_answer 154) Osteoporosis, an age-related disease of skeletal system, may occur due to
A)
Immune disorder affecting neuromuscular junction leading to fatigue
done
clear
B)
High concentration of \[C{{a}^{++}}\] and \[N{{a}^{+}}\]
done
clear
C)
Decreased level of estrogen
done
clear
D)
Accumulation of uric acid leading to inflammation of joints
done
clear
View Answer play_arrow
question_answer 155) Serum differs from blood in
A)
Lacking globulins
done
clear
B)
Lacking albumins
done
clear
C)
Lacking clotting factors
done
clear
D)
Lacking antibodies
done
clear
View Answer play_arrow
question_answer 156) Lungs do not collapse between breaths and some air always remains in the lungs which can never be expelled because
A)
There is a negative pressure in the lungs
done
clear
B)
There is a negative intrapleural pressure pulling at the lung walls
done
clear
C)
There is a positive intrapleural pressure
done
clear
D)
Pressure in the lungs is higher than the atmospheric pressure
done
clear
View Answer play_arrow
question_answer 157) The posterior pituitary gland is not a 'true' endocrine gland because
A)
It is provided with a duct
done
clear
B)
It only stores and releases hormones
done
clear
C)
It is under the regulation of hypothalamus pressure pulling at the lung walls.
done
clear
D)
It secretes enzymes
done
clear
View Answer play_arrow
question_answer 158) The part of nephron involved in active reabsorption of sodium is
A)
Distal convoluted tubule
done
clear
B)
Proximal convoluted tubule
done
clear
C)
Bowman's capsule
done
clear
D)
Descending limb of Henle's loop
done
clear
View Answer play_arrow
question_answer 159) Which of the following is hormone releasing IUD?
A)
LNG-20
done
clear
B)
Multiload-375
done
clear
C)
Lippes loop
done
clear
D)
Cu7
done
clear
View Answer play_arrow
question_answer 160) Which of the following is incorrect regarding vasectomy?
A)
No sperm occurs in seminal fluid
done
clear
B)
No sperm occurs in epididymis
done
clear
C)
Vasa deferentia is cut and tied
done
clear
D)
Irreversible sterility
done
clear
View Answer play_arrow
question_answer 161) Embryo with more than 16 blastomeres formed due to in-vitro fertilization is transferred into
A)
Uterus
done
clear
B)
Fallopian tube
done
clear
C)
Fimbriae
done
clear
D)
Cervix
done
clear
View Answer play_arrow
question_answer 162) Which of the following depicts the correct pathway of transport of sperms?
A)
Rete testis \[\to \] Efferent ductules \[\to \] Epididymis \[\to \] Vas deferens
done
clear
B)
Rate testis \[\to \] Epididymis \[\to \] Efferent ductules \[\to \] Vas deferens
done
clear
C)
Rete testis \[\to \] Vas deferens \[\to \] Efferent ductules \[\to \] Epididymis
done
clear
D)
Efferent ductules \[\to \] Rete testis \[\to \] Vas deferens \[\to \] Epididymis
done
clear
View Answer play_arrow
question_answer 163)
Match Column - I with Column - II and select the correct option using the codes given below:
Column - I
Column - II
A. Mons pubis
(i) Embryo formation
B. Antrum
(ii) Sperm
C. Trophectoderm
(iii) Female external genitalia
D. Nebenkern
(iv) Graafian follicle
A)
A - (iii) B - (iv) C - (ii) D - (i)
done
clear
B)
A - (iii) B - (iv) C - (i) D - (ii)
done
clear
C)
A - (iii) B - (i) C - (iv) D - (ii)
done
clear
D)
A - (i) B - (iv) C - (iii) D - (ii)
done
clear
View Answer play_arrow
question_answer 164) Several hormones like hCG, hPL, estrogen, progesterone are produced by
A)
Ovary
done
clear
B)
Placenta
done
clear
C)
Fallopian tube
done
clear
D)
Pituitary
done
clear
View Answer play_arrow
question_answer 165) If a colour-blind man marries a woman who is homozygous for normal colour vision, the probability of their son being colour-blind is
A)
0
done
clear
B)
0.5
done
clear
C)
0.75
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 166) Genetic drift operates in
A)
Small isolated population
done
clear
B)
Large isolated population
done
clear
C)
Non-reproductive population
done
clear
D)
Slow reproductive population
done
clear
View Answer play_arrow
question_answer 167) In Hardy-Weinberg equation, the frequency of heterozygous individual is represented by
A)
\[{{p}^{2}}\]
done
clear
B)
\[2pq\]
done
clear
C)
\[pq\]
done
clear
D)
\[{{q}^{2}}\]
done
clear
View Answer play_arrow
question_answer 168) The chronological order of human evolution from early to the recent is
A)
Australopithecus \[\to \] Ramapithecus \[\to \] Homo habilis \[\to \] Homo erectus
done
clear
B)
Ramapithecus \[\to \] Australopithecus \[\to \] Homo habilis \[\to \] Homo erectus
done
clear
C)
Ramapithecus \[\to \] Homo habilis \[\to \] Australopithecus \[\to \] Homo erectus
done
clear
D)
Australopithecus \[\to \] Homo habilis \[\to \] Ramapithecus \[\to \] Homo erectus
done
clear
View Answer play_arrow
question_answer 169)
Which of the following is the correct sequence of events in the origin of life? (i) Formation of protobionts (ii) Synthesis of organic monomers (iii) Synthesis of organic polymers (iv) Formation of DNA-based genetic systems
A)
(i) (ii) (iii) (iv)
done
clear
B)
(i) (iii) (ii) (iv)
done
clear
C)
(ii) (iii) (i) (iv)
done
clear
D)
(ii) (iii) (iv) (i)
done
clear
View Answer play_arrow
question_answer 170) A molecule that can act as a genetic material must fulfill the traits given below, except
A)
It should be able to express itself in the form of 'Mendelian characters'
done
clear
B)
It should be able to generate its replica
done
clear
C)
It should be unstable structurally and chemically
done
clear
D)
It should provide the scope for slow changes that are required for evolution
done
clear
View Answer play_arrow
question_answer 171) DNA-dependent RNA polymerase catalyzes transcription on one strand of the DNA which is called the
A)
Template strand
done
clear
B)
Coding strand
done
clear
C)
Alpha strand
done
clear
D)
Antistrand
done
clear
View Answer play_arrow
question_answer 172) Interspecific hybridization is the mating of
A)
Animals within same breed without having common ancestors
done
clear
B)
Two different related species
done
clear
C)
Superior males and females of different breeds
done
clear
D)
More closely related individuals within same breed for \[4-6\] generations
done
clear
View Answer play_arrow
question_answer 173) Which of the following is correct regarding AIDS causative agent HIV?
A)
HIV is enveloped virus containing one molecule of single-stranded RNA and one molecule of reverse transcriptase
done
clear
B)
HIV is enveloped virus that contains two identical molecules of single-stranded RNA and two molecules of reverse transcriptase
done
clear
C)
HIV is unenveloped retrovirus
done
clear
D)
HIV does not escape but attacks the acquired immune response
done
clear
View Answer play_arrow
question_answer 174) Among the following edible fishes, which one is a marine fish having rich source of omega-3 fatty acids?
A)
Mystus
done
clear
B)
Mangur
done
clear
C)
Mrigala
done
clear
D)
Mackerel
done
clear
View Answer play_arrow
question_answer 175)
Match Column-I with Column-II and select the correct option using the codes given below :
Column - I
Column - II
A. Citric acid
(i) Trichoderma
B. Cyclosporin A
(ii) Clostridium
C. Statins
(iii) Aspergillus
D. Butyric acid
(iv) Monascus
A)
A - (iii) B - (i) C - (ii) D - (iv)
done
clear
B)
A - (iii) B - (i) C - (iv) D - (ii)
done
clear
C)
A - (i) B - (iv) C - (ii) D - (iii)
done
clear
D)
A - (iii) B - (iv) C - (i) D - (ii)
done
clear
View Answer play_arrow
question_answer 176) Biochemical Oxygen Demand (BOD) may not be a good index for pollution for water bodies receiving effluents from
A)
Domestic sewage
done
clear
B)
Dairy industry
done
clear
C)
Petroleum industry
done
clear
D)
Sugar industry
done
clear
View Answer play_arrow
question_answer 177) The principle of competitive exclusion was stated by
A)
C. Darwin
done
clear
B)
G. F. Gause
done
clear
C)
MacArthur
done
clear
D)
Verhulstand Pearl
done
clear
View Answer play_arrow
question_answer 178) Which of the following National Parks is home to the famous musk deer or hangul?
A)
Keibul Lamjao National Park, Manipur
done
clear
B)
Bandhavgarh National Park, Madhya Pradesh
done
clear
C)
Eaglenest Wildlife Sanctuary, Arunachal Pradesh
done
clear
D)
Dachigam National Park, Jammu & Kashmir
done
clear
View Answer play_arrow
question_answer 179) A lake which is rich in organic waste may result in
A)
Increased population of aquatic organisms due to minerals
done
clear
B)
Drying of the lake due to algal bloom
done
clear
C)
Increased population offish due to lots of nutrients
done
clear
D)
Mortality of fish due to lack of oxygen
done
clear
View Answer play_arrow
question_answer 180) The highest DDT concentration in aquatic food chain shall occur in
A)
Phytop lankton
done
clear
B)
Seagull
done
clear
C)
Crab
done
clear
D)
Eel
done
clear
View Answer play_arrow