question_answer 1) A car is moving at a speed of 72 km/hr. The diameter of its wheels is 0.5 m. If the wheels are stopped in 20 rotations applying brakes. Then angular retardation provided by the brake is:
A)
\[-45.\,5\,rad/{{s}^{2}}\]
done
clear
B)
\[-33.\,5\,rad/{{s}^{2}}\]
done
clear
C)
\[-29.\,5\,rad/{{s}^{2}}\]
done
clear
D)
\[-25.\,5\,rad/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2) A bullet is fired from the gun with a speed of 1000 m/s in order to hit a target s = 100 in away. At what height above the target should by gun be aimed (The resistance of air is negligible and \[g=10\,m/{{s}^{2}}\])
A)
23 cm
done
clear
B)
15 cm
done
clear
C)
9 cm
done
clear
D)
5 cm
done
clear
View Answer play_arrow
question_answer 3) If the coefficient of friction of a plane inclined at \[45{}^\circ \] is 0.5. Then acceleration of a body sliding freely on it will be:
A)
\[\frac{9.8}{2\sqrt{2}}m/{{s}^{2}}\]
done
clear
B)
\[\frac{9.8}{\sqrt{2}}m/{{s}^{2}}\]
done
clear
C)
\[9.8\,m/{{s}^{2}}\]
done
clear
D)
\[4.8\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 4) Assuming earth to be sphere of uniform density what is the value of acceleration due to gravity at a point 100 km below the earth surface (Given \[R=6380\,\times {{10}^{3}}\,m\])
A)
3.10 m/s
done
clear
B)
\[5.06\,m/{{s}^{2}}\]
done
clear
C)
\[7.64\,m/{{s}^{2}}\]
done
clear
D)
\[9.66\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 5) Plancks constant has same dimension as:
A)
angular momentum
done
clear
B)
linear momentum
done
clear
C)
force
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 6) The mass of ship is \[2\times {{10}^{7}}\,kg\]. On applying a fore of \[25\times {{10}^{5}}N,\] it is displaced through 25 m. After the displacement. The speed a required by the ship will be:
A)
12.5 m/s
done
clear
B)
5 m/s
done
clear
C)
3.7 m/s
done
clear
D)
2.5 m/s
done
clear
View Answer play_arrow
question_answer 7) A 500 kg car takes around turn of radius 50 m with a speed of 36 km/hr. The centripetel force acting on the car will be:
A)
1200 N
done
clear
B)
1000 N
done
clear
C)
750 N
done
clear
D)
250 N
done
clear
View Answer play_arrow
question_answer 8) An apple gives 21 kJ energy to a boy. How much height he can climb by using this energy. If his efficiency is 28%? (mass of boy 40 kg)
A)
22.5 m
done
clear
B)
15 m
done
clear
C)
10m
done
clear
D)
5m
done
clear
View Answer play_arrow
question_answer 9) The weight of an object at earths surface is 700 g wt. What will be its weight at the surface of a planet whose radius is ½ and mass is 1/7 of that of the earth?
A)
200 g wt.
done
clear
B)
400 g wt.
done
clear
C)
50 g wt.
done
clear
D)
300 g wt.
done
clear
View Answer play_arrow
question_answer 10) Out of the given bodies (of same mass) for which the moment of inertia will be maximum about the axis passing through its centre of gravity and perpendicular to its plane?
A)
Disc of radius\[a\]
done
clear
B)
Ring of radius\[a\]
done
clear
C)
Square lamina of side\[2a\]
done
clear
D)
Pour rods of length \[2a\] making a square
done
clear
View Answer play_arrow
question_answer 11) A moving body of mass in and velocity 3 km/hr. Collides with a body at rest and of mass 2 in and then sticks to it. Now the combined mass starts to move, then the combined velocity will be:
A)
4 km/hr
done
clear
B)
3 km/hr
done
clear
C)
2 km/hr
done
clear
D)
1 km/hr
done
clear
View Answer play_arrow
question_answer 12) A 120m long train is moving towards west with a speed of 10 m/s. A bird flying towards east with a speed of 3 m/s crosses the train. The time taken by the bird to cross the train will be:
A)
16 sec
done
clear
B)
12 sec
done
clear
C)
10 sec
done
clear
D)
8sec
done
clear
View Answer play_arrow
question_answer 13) The velocity of all radio waves in free space is \[3\times {{10}^{5}}\,m/s\]. The frequency of a radio wave of wavelength 150 m is:
A)
20 kHz
done
clear
B)
2 kHz
done
clear
C)
2 MHz
done
clear
D)
1 MHz
done
clear
View Answer play_arrow
question_answer 14) A boy of 50 kg is standing in a left moving down with acceleration \[9.8\,m/{{s}^{2}}\]. The apparent weight of the boy is:
A)
\[\frac{50}{9.8}N\]
done
clear
B)
\[50\times 9.8\,N\]
done
clear
C)
50 N
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 15) A particle executing SHM has amplitude and frequency 60 Hz. The maximum acceleration of the particle is:
A)
\[60{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
B)
\[88\,{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
C)
\[140\,{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
D)
\[144\,{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) If the equation of motion of standing Waves is y = 0.3 sin (314 t - 1.57 x), then the velocity of standing wave will be:
A)
400 m/s
done
clear
B)
300 m/s
done
clear
C)
200 m/s
done
clear
D)
100 m/s
done
clear
View Answer play_arrow
question_answer 17) In a thermodynamics process pressure of a fixed mass of a gas is changed in such a manner that the gas molecule give out 30 joule of heat and 10 joule of work is done on the gas. If the initial internal energy of the gas was 40 joule, then the final internal energy will be:
A)
-20 J
done
clear
B)
20 J
done
clear
C)
80 J
done
clear
D)
3 J
done
clear
View Answer play_arrow
question_answer 18) A black body is heated from \[{{27}^{o}}C\] to \[{{927}^{o}}C\] the ratio of radiations emitted will be:
A)
1 : 256
done
clear
B)
1 : 64
done
clear
C)
1 : 16
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 19) The efficiency of a Carnot engine operating with reservoir temperature of \[100{}^\circ C\]and \[-23{}^\circ C\] will be:
A)
\[\frac{100-23}{373}\]
done
clear
B)
\[\frac{100+23}{373}\]
done
clear
C)
\[\frac{100+23}{100}\]
done
clear
D)
\[\frac{100-23}{100}\]
done
clear
View Answer play_arrow
question_answer 20) A body cools from \[60{}^\circ C\] to \[50{}^\circ C\] in 10: minutes. If the room temperature is \[25{}^\circ C\] and assuming newtons law of cooling to hold good, the temperature of the body at the end of the next 10 minutes will be:
A)
\[25{}^\circ C\]
done
clear
B)
\[42.85{}^\circ C\]
done
clear
C)
\[40{}^\circ C\]
done
clear
D)
\[38.5{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 21) A circular coil of radius 2 cm has 10 turns, if 0.5 A current is flowing through it, then what will be the magnetic field at its centre?
A)
\[1.57\times {{10}^{6}}T\]
done
clear
B)
\[1.57\times {{10}^{-5}}T\]
done
clear
C)
\[1.57\times {{10}^{-4}}T\]
done
clear
D)
\[1.57T\]
done
clear
View Answer play_arrow
question_answer 22) A galvanometer can be changed into ammeter by connecting:
A)
high resistance in parallel
done
clear
B)
high resistance in series
done
clear
C)
low resistance in parallel
done
clear
D)
low resistance in series
done
clear
View Answer play_arrow
question_answer 23) A prism has a refracting angle \[60{}^\circ \]. A ray of given monochromatic light suffers minimum deviation of \[38{}^\circ \] in passing through prism refractive index of the material of prism is:
A)
1.5094
done
clear
B)
1.3056
done
clear
C)
0.7849
done
clear
D)
2.425
done
clear
View Answer play_arrow
question_answer 24) A metal coin is at the bottom of a beaker filled with a liquid of refractive index 4/3 to height of 6 cm. To an observer looking from above the surface of the liquid, coin will appear at a depth of:
A)
7.5 cm
done
clear
B)
6.75 cm
done
clear
C)
4.5 cm
done
clear
D)
1.5 cm
done
clear
View Answer play_arrow
question_answer 25) An astronomical telescope has two lenses of focal powers 0.5 D and 20 D. Then its magnifying power will be:
A)
8
done
clear
B)
20
done
clear
C)
30
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 26) The peak value of AC voltage on a 220V mains is:
A)
\[240\sqrt{2}V\]
done
clear
B)
\[230\sqrt{2}V\]
done
clear
C)
\[220\sqrt{2}V\]
done
clear
D)
\[200\sqrt{2}V\]
done
clear
View Answer play_arrow
question_answer 27) A resistance of a galvanometer\[G=6\Omega \] maximum current of 2 amp is measured by it. Then required resistance to convert it into ail ammeter reading up to 6 A, will of:
A)
5\[5\,\Omega \]
done
clear
B)
\[4\,\Omega \]
done
clear
C)
\[3\,\Omega \]
done
clear
D)
\[2\,\Omega \]
done
clear
View Answer play_arrow
question_answer 28) Two copper wires are of same length one of the twice as thick as the other. Then the resistance of the two wires are in the ratio of:
A)
1 : 16
done
clear
B)
1 : 8
done
clear
C)
1 : 4
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 29) A hot electric iron has a resistance of 80\[\,\Omega \] and is used on a 200 V source. The electrical energy spent, if it is used for hr, will be:
A)
8000 Wh
done
clear
B)
2000 Wh
done
clear
C)
1000 Wh
done
clear
D)
800Wh
done
clear
View Answer play_arrow
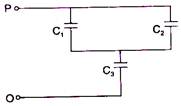
question_answer 30)
In the given network capacitance \[{{C}_{2}}=10\,\mu F,\] \[{{C}_{1}}=5\,\mu F\] and \[{{C}_{3}}=4\,\mu F.\] The resultant capacitance between P and Q will be:
A)
\[4.7\,\mu F\]
done
clear
B)
\[1.2\,\mu F\]
done
clear
C)
\[3.2\,\mu F\]
done
clear
D)
\[2.2\,\mu F\]
done
clear
View Answer play_arrow
question_answer 31) The no. of turns in primary and secondary of a transformer are 5 and 10 and mutual inductance of transformer is 25 henry. Now, the no. of turns in primary and secondary are 10 and 5, the new mutual inductance will be:
A)
6.25 H
done
clear
B)
12.5H
done
clear
C)
25 H
done
clear
D)
50 H
done
clear
View Answer play_arrow
question_answer 32) From a point charge, there is a fixed point A. At A, there is an electric field of 500 volt/meter and potential difference of 3000 V. Distance between the point charge and A is
A)
24 m
done
clear
B)
16 m
done
clear
C)
12 m
done
clear
D)
6 m
done
clear
View Answer play_arrow
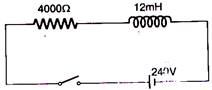
question_answer 33)
In the inductive circuit given in the figure, the currents rises after the switch is closed. At instant when the current is 15 mA, then potential difference across the inductor will be:
A)
zero
done
clear
B)
240 V
done
clear
C)
180 V
done
clear
D)
60 V
done
clear
View Answer play_arrow
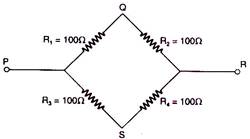
question_answer 34)
From resistances of 100 ohm each are connected in the form of a square. The effective resistance along the diagonal points P R. is:
A)
\[100\,\Omega \]
done
clear
B)
\[180\,\Omega \]
done
clear
C)
\[220\,\Omega \]
done
clear
D)
\[440\,\Omega \]
done
clear
View Answer play_arrow
question_answer 35) 700 pF capacitor is charged by 50 V battery. Electrostatic energy is stored by it will be:
A)
\[17.0\times {{10}^{-8}}J\]
done
clear
B)
\[13.0\times {{10}^{-9}}J\]
done
clear
C)
\[8.7\times {{10}^{-7}}J\]
done
clear
D)
\[6.7\times {{10}^{-7}}J\]
done
clear
View Answer play_arrow
question_answer 36) An aeroplane having a wing space of 35 m flies due north with the speed of 90 m/s given \[B\,4\times {{10}^{-5}}\]tesla. The potential difference between the tips of the wings will be:
A)
0.013V
done
clear
B)
1.26V
done
clear
C)
12.6V
done
clear
D)
0.126V
done
clear
View Answer play_arrow
question_answer 37) Two coils have mutual inductance 0.005 H. The current changes in the first coil according to equation\[I={{I}_{0}}\] sin cot. Where \[{{I}_{0}}=10\] amp and \[\omega =10\,\pi \] rad/sec. The maximum value of emf in the second coil is:
A)
\[12\,\pi \]
done
clear
B)
\[8\,\pi \]
done
clear
C)
\[5\,\pi \]
done
clear
D)
\[2\,\pi \]
done
clear
View Answer play_arrow
question_answer 38) A radioactive element has half life of 3.6 days. In what time will it be left 1/32nd undecayed?
A)
4 days
done
clear
B)
12 days
done
clear
C)
18 days
done
clear
D)
24 days
done
clear
View Answer play_arrow
question_answer 39) In a \[\text{p-}\]type semiconductor germanium is doped with:
A)
gallium
done
clear
B)
aluminium
done
clear
C)
boron
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 40) The wavelength associated with an electron accelerated through a potential difference of 100 V is of the order of:
A)
\[1.2\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[10.5\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[100\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 41) A body whose moment of inertia is 3 kg-m2 is in rest. It is rotated for 20 sec by a torque of 6 Nm, angular displacement of the body will be:
A)
400 radian
done
clear
B)
200 radian
done
clear
C)
100 radian
done
clear
D)
250 radian
done
clear
View Answer play_arrow
question_answer 42) A straight conductor of length 4 m moves at a speed of 10 m/s. When the conductor makes an angle of \[30{}^\circ \] with the direction of magnetic field of induction of 0.1 wb.perm2 then induced emf. is:
A)
8 V
done
clear
B)
4 V
done
clear
C)
1 V
done
clear
D)
2 V
done
clear
View Answer play_arrow
question_answer 43) The work function for aluminium surface is 4.2eV. The cutoff wavelength for photoelectric effect is:
A)
\[1000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2955\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[4200\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 44) The ionization potential of hydrogen atom is 13.6 volt. In the lowest energy level, this atom is ionized by absorbing a photon of \[800\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The kinetic energy of the released electron will be:
A)
15.51 eV
done
clear
B)
2.91 eV
done
clear
C)
13.6 eV
done
clear
D)
1.91 eV
done
clear
View Answer play_arrow
question_answer 45) Penetrating power is minimum for:
A)
X-rays
done
clear
B)
\[\text{-}\]rays
done
clear
C)
\[\text{-}\]rays
done
clear
D)
\[\text{-}\]rays
done
clear
View Answer play_arrow
question_answer 46) The functions of moderators in nuclear reactor is to:
A)
decrease the speed of neutrons
done
clear
B)
increase the speed of neutrons
done
clear
C)
decrease the speed of electrons
done
clear
D)
increase the speed of electrons
done
clear
View Answer play_arrow
question_answer 47) A chain reaction in fission of uranium is possible, because:
A)
two intermediate sized nuclear fragments are formed
done
clear
B)
three neutrons are given out in each fission
done
clear
C)
fragments in fission are radioactive
done
clear
D)
large amount of energy is released
done
clear
View Answer play_arrow
question_answer 48) A nuclei X with mass number A and charge number Z, disintegrates into one \[\alpha \text{-}\]particle and one (\[\beta \text{-}\]particle. The resulting nuclide R has atomic mass and atomic number, equal to:
A)
(A - Z) and (Z - 1)
done
clear
B)
(A- Z) and (Z- 2)
done
clear
C)
(A - 4) and (A - 2)
done
clear
D)
A - 4) and (Z- 1)
done
clear
View Answer play_arrow
question_answer 49) Two inductors each of inductance L are joined in parallel. Their equivalent inductance will be:
A)
zero
done
clear
B)
\[\frac{L}{2}\]
done
clear
C)
L
done
clear
D)
2 L
done
clear
View Answer play_arrow
question_answer 50) What will be the amount of energy absorbed when an electron jumps from first orbit to second orbits? (If the value of energy in nth orbit of H-atom is expressed as \[{{E}_{n}}=-\frac{13.6}{{{n}^{2}}}eV)\]
A)
- 3.4 eV
done
clear
B)
- 6.6 eV
done
clear
C)
-8.1eV
done
clear
D)
-10.2eV
done
clear
View Answer play_arrow
question_answer 51) The velocity of bullet is reduced from 200 m/s to 100 m/s while travelling through a wooden block of thickness of 10 cm. The retardation assuming to be uniform, will be:
A)
\[15\times {{10}^{4}}m/{{s}^{2}}\]
done
clear
B)
\[13.5\times {{10}^{4}}m/{{s}^{2}}\]
done
clear
C)
\[12\times {{10}^{4}}m/{{s}^{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 52) A stone tied to one end of spring 80 cm long is whirled in a horizontal circle with a constant speed. If stone makes25 revolutions in 14 sec. the magnitude of acceleration of stone is:
A)
\[850\,cm/{{s}^{2}}\]
done
clear
B)
\[996\,cm/{{s}^{2}}\]
done
clear
C)
\[720\,cm/{{s}^{2}}\]
done
clear
D)
\[650\,cm/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 53) A concave mirror of focal length 15 cm forms an image having twice the linear dimension of the object. The position of the object when the image is virtual will be:
A)
45 cm
done
clear
B)
30 cm
done
clear
C)
7.5 cm
done
clear
D)
22.5 cm
done
clear
View Answer play_arrow
question_answer 54) The thermodynamic co-ordinates of a jar filled with gas A are P. V and T and another jar B filled with another gas are IP, V/4 and 2T, where the symbols have their usual meaning. The ratio of the number of molecules of jar A to those of jar B is:
A)
4 : 1
done
clear
B)
2 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 55) If the pressure of a gas contained in a vessel is increased by 0.4% when heated through 1\[{}^\circ C\], the initial temperature had been:
A)
2500 K
done
clear
B)
250 K
done
clear
C)
\[250{}^\circ C\]
done
clear
D)
\[25{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 56) A square loop of side L carries current t, magnetic field at the centre of the loop:
A)
does not depend on L
done
clear
B)
directly proportional to \[{{L}^{2}}\]
done
clear
C)
inversly proportional to L
done
clear
D)
directly proportional to L
done
clear
View Answer play_arrow
question_answer 57) For a given angle of projection, if the time of flight of a projectile is doubled the horizontal range will increase to:
A)
four times
done
clear
B)
thrice
done
clear
C)
once
done
clear
D)
twice
done
clear
View Answer play_arrow
question_answer 58) When the kinetic energy of a body executing S.H.M. is 1/3 of the potential energy. The displacement of the body is x percent of the amplitude, where x is:
A)
33
done
clear
B)
87
done
clear
C)
67
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 59) In the case of horse pulling a cart, the force that causes the horse to move forward is the force that:
A)
the horse exerts on the ground
done
clear
B)
the horse exerts on the cart
done
clear
C)
the ground exerts on the horse
done
clear
D)
the cart exerts on the horse
done
clear
View Answer play_arrow
question_answer 60) If retardation produced by air resistance of projectile is one-tenth of acceleration due to gravity, the lime to reach maximum height:
A)
decreases by 11 percent
done
clear
B)
increases by 11 percent
done
clear
C)
decreases by 90 percent
done
clear
D)
increases by 90 percent
done
clear
View Answer play_arrow
question_answer 61) y component of velocity is 20 and x component of velocity is 10. The direction of motion of the body with the horizontal at this instant is:
A)
\[{{\tan }^{-1}}(2)\]
done
clear
B)
\[{{\tan }^{-1}}(1/2)\]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[0{}^\circ \]
done
clear
View Answer play_arrow
question_answer 62) The dimensional formula of light year is:
A)
\[[{{L}^{-1}}]\]
done
clear
B)
\[[{{T}^{-1}}]\]
done
clear
C)
\[[L]\]
done
clear
D)
\[[T]\]
done
clear
View Answer play_arrow
question_answer 63) In a good conductor of electricity, the type of bonding that exists is:
A)
ionic
done
clear
B)
van der waal
done
clear
C)
covalent
done
clear
D)
metallic
done
clear
View Answer play_arrow
question_answer 64) Two identical straight wires are stretched so as to produce 6 beats per second when vibrating simultaneously. On changing the tension slightly in one of them, the beat frequency stilt remains unchanged. Denoting by \[{{T}_{1}}\]and \[{{T}_{2,}}\] the higher and the lower initial tensions in the strings, it could be said that while making the above changes in tension:
A)
\[{{T}_{1}}\]was decreased
done
clear
B)
\[{{T}_{1}}\]was increased
done
clear
C)
\[{{T}_{2}}\] was increased
done
clear
D)
\[{{T}_{2}}\] was decreased
done
clear
View Answer play_arrow
question_answer 65) When beats are produced by two progressive waves of the same amplitude and of nearly the same frequency, the ratio of maximum loudness to the loudness of one of the waves will be n. Where n is:
A)
3
done
clear
B)
1
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 66) A body is suspended from a smooth horizontal nail by a string of length 0.25 metre. What minimum horizontal velocity should be given to it in the lowest position so that it may move in a complete vertical circle with the nail at the centre?
A)
\[\sqrt{12.25}\,m{{s}^{-1}}\]
done
clear
B)
\[4.9\,m{{s}^{-1}}\]
done
clear
C)
\[7\sqrt{2}\,m{{s}^{-1}}\]
done
clear
D)
\[\sqrt{9.8}\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 67) Two rain drops falling through air have radii in the ratio 1 : 2. They will have terminal velocity in the ratio:
A)
4 : 1
done
clear
B)
1 : 4
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 68) A body is acted on by force towards a point. The magnitude of the force is inversely proportional to the square of the distance. The path of body will be:
A)
ellipse
done
clear
B)
hyperbola
done
clear
C)
circle
done
clear
D)
parabola
done
clear
View Answer play_arrow
question_answer 69) An electric immersion heater of 1.08 kW is immersed in water. After the water has readied a temperature of\[100{}^\circ C\], how much time will be required to produce 100 got steam?
A)
210 s
done
clear
B)
105 s
done
clear
C)
420 s
done
clear
D)
50 s
done
clear
View Answer play_arrow
question_answer 70) The speed of sound through oxygen at TK is \[\upsilon \,m{{s}^{-1}}\]. As the temperature becomes 2T and oxygen gas dissociates into atomic oxygen, the speed of sound:
A)
remains the same
done
clear
B)
becomes \[2\upsilon \]
done
clear
C)
becomes \[\sqrt{2\upsilon }\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 71) Illumination of the sun at noon is maximum because:
A)
scattering is reduced at noon
done
clear
B)
refraction of light is minimum at noon
done
clear
C)
rays are incident almost normally
done
clear
D)
the sun is nearer to earth at noon
done
clear
View Answer play_arrow
question_answer 72) Two spheres of radii in the ratio 1 : 2 and densities in the ratio 2 : 1 and of same specific heat, are heated to same temperature and left in the same surrounding. Their rate of cooling will be in the ratio :
A)
2 : 1
done
clear
B)
1 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 73) The formation of ice is started in a lake with water at \[0{}^\circ C\]. When the atmospheric temperature is -\[10{}^\circ C\]. If time taken for 1 cm of ice to be formed is 7 hours, the time taken for the thickness of ice to increase from 1 cm to cm is:
A)
less than 7 hours
done
clear
B)
7 hours
done
clear
C)
more than 14 hours
done
clear
D)
more than 7 hours but less than 14 hours
done
clear
View Answer play_arrow
question_answer 74) If an ideal flask containing hot coffee is shaken, the temperature of the coffee will:
A)
decrease
done
clear
B)
increase
done
clear
C)
remain same
done
clear
D)
decrease if temperature is below \[4{}^\circ C\] and increase if temperature is equal to or more than \[4{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 75) Water is used to cool the radiators of engines in cars because:
A)
of its low boiling point
done
clear
B)
of its high specific heat
done
clear
C)
of its low density
done
clear
D)
of its easy availability
done
clear
View Answer play_arrow
question_answer 76) Suppose radius of the moons orbit around the earth is doubled. Its period around the earth will become :
A)
1/2 times
done
clear
B)
\[\sqrt{2}\] times
done
clear
C)
\[{{2}^{2/3}}\] times
done
clear
D)
\[{{2}^{3/2}}\] times
done
clear
View Answer play_arrow
question_answer 77) A sphere is accelerated upwards by a cord whose braking strength is four times its weight. The maximum acceleration with which the sphere can move up without breaking the cord is:
A)
g
done
clear
B)
3g
done
clear
C)
2g
done
clear
D)
4g
done
clear
View Answer play_arrow
question_answer 78) The potential due to dipole at a point is proportional to:
A)
\[{{r}^{-2}}\]
done
clear
B)
\[{{r}^{3}}\]
done
clear
C)
\[{{r}^{2}}\]
done
clear
D)
r
done
clear
View Answer play_arrow
question_answer 79) The capacitance of a spherical conductor with radius 1 m is:
A)
\[9\times {{10}^{-9}}F\]
done
clear
B)
\[1\mu F\]
done
clear
C)
\[2.5\times {{10}^{-10}}F\]
done
clear
D)
\[1\times {{10}^{-6}}F\]
done
clear
View Answer play_arrow
question_answer 80) A body moves for a total of nine second starting from rest with uniform acceleration and then with uniform retardation, which is twice the value of acceleration and then stops. The duration of uniform acceleration is:
A)
3 s
done
clear
B)
4.5 s
done
clear
C)
5s
done
clear
D)
6 s
done
clear
View Answer play_arrow
question_answer 81) The magnifying power of objective of a compound microscope is 7. If magnifying power of microscope is 35, the magnifying power of eye lens will be:
A)
5
done
clear
B)
30
done
clear
C)
35
done
clear
D)
28
done
clear
View Answer play_arrow
question_answer 82) A convex lens makes a real image of 4 cm long on a screen. When the km is shifted to a new position without disturbing the object or the screen, we get a real image on the screen, which is 16 cm long. The length of the object is:
A)
1/4 cm
done
clear
B)
8 cm
done
clear
C)
20 cm
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 83) The displacement equation for a progressive wave is \[y=5\times {{10}^{-5}}\] sin (100 t-50 x), where quantities are in MKS system, velocity of wave will be:
A)
1 m/s
done
clear
B)
2 m/s
done
clear
C)
0.5 m/s
done
clear
D)
15 m/s
done
clear
View Answer play_arrow
question_answer 84) Bernoullis principle is based on the law of conservation of:
A)
angular momentum
done
clear
B)
linear momentum
done
clear
C)
mass
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 85) The colour of a star indicates its:
A)
temperature
done
clear
B)
distance
done
clear
C)
velocity
done
clear
D)
size
done
clear
View Answer play_arrow
question_answer 86) Efficiency of a half-wave rectifier is nearly:
A)
80%
done
clear
B)
60%
done
clear
C)
40%
done
clear
D)
20%
done
clear
View Answer play_arrow
question_answer 87) The current ill a diode is related to the voltage V by the equation:
A)
\[I\propto {{V}^{1/2}}\]
done
clear
B)
\[I\propto {{V}^{3/2}}\]
done
clear
C)
\[I\propto {{V}^{2}}\]
done
clear
D)
\[I\propto V\]
done
clear
View Answer play_arrow
question_answer 88) An X-ray photon has a wavelength \[0.01\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. Its momentum (in \[kg\,m{{s}^{-1}}\]) is:
A)
\[6.66\,\times {{10}^{-22}}\]
done
clear
B)
\[3.3\,\times {{10}^{-32}}\]
done
clear
C)
\[6.6\times {{10}^{-22}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 89) The equation \[{{4}_{1}}{{H}^{1}}{{\xrightarrow{{}}}_{2}}H{{e}^{4++}}+2{{e}^{+}}+26\,MeV\]represents:
A)
\[\text{-}\]decay
done
clear
B)
\[\beta \text{-}\] decay
done
clear
C)
fission
done
clear
D)
fusion
done
clear
View Answer play_arrow
question_answer 90)
An infinitely long straight conductor is bent into the shape as shown in figure. It carries a current (A and radius of the circular loop is r m. The magnetic field at the centre of the loop is:
A)
\[{{\mu }_{0i}}(\pi -1)/2\pi r\]
done
clear
B)
\[{{\mu }_{0i}}(\pi +1)/2\pi r\]
done
clear
C)
\[{{\mu }_{i}}/2\pi r\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 91) According to Newton viscus force is given by \[F=-\eta A\frac{d\upsilon }{dx}\]where\[\eta =\]coefficient of viscosity, so dimensions of \[\eta \] will be:
A)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
B)
\[[ML{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{-1}}{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 92) A truck travelling due north at \[20\,m{{s}^{-1}}\] turns west and travels with same speed. What is the change in velocity?
A)
\[20\sqrt{2}\,m{{s}^{-1}}\] south-west
done
clear
B)
\[40\,m{{s}^{-1}}\] south-west
done
clear
C)
\[20\sqrt{2}\,m{{s}^{-1}}\] north-west
done
clear
D)
\[40\,m{{s}^{-1}}\] north-west
done
clear
View Answer play_arrow
question_answer 93) An iron bar of length \[l\] and having a cross-section A is heated from 0 to \[100{}^\circ C\]. If this bar is so held that it is not permitted to expand or bend/ the force that is developed, is:
A)
inversely proportional to the cross-sectional area of the bar
done
clear
B)
independent of the length of the bar
done
clear
C)
inversely proportional to the length of the bar
done
clear
D)
directly proportional to the length of the bar
done
clear
View Answer play_arrow
question_answer 94) A body of uniform cross-sectional area floats in a liquid of density thrice its value. The fraction of exposed height will be:
A)
\[\frac{2}{3}\]
done
clear
B)
\[\frac{5}{6}\]
done
clear
C)
\[\frac{1}{6}\]
done
clear
D)
\[\frac{1}{3}\]
done
clear
View Answer play_arrow
question_answer 95) The restoring force of SHM is maximum when particle :
A)
displacement is maximum
done
clear
B)
half way between them
done
clear
C)
when crossing mean position
done
clear
D)
at rest
done
clear
View Answer play_arrow
question_answer 96) Two forces of UN and 8N act upon a body. The resultant force on the body has a maximum Value of :
A)
4 N
done
clear
B)
0 N
done
clear
C)
20 N
done
clear
D)
8 N
done
clear
View Answer play_arrow
question_answer 97) Which of following pairs does not have similar dimensions?
A)
Plancks constant and angular momentum
done
clear
B)
Tension and surface tension
done
clear
C)
Angle and strain
done
clear
D)
Stress and pressure
done
clear
View Answer play_arrow
question_answer 98) Four tenses arc made from the same type of glass. The radius of curvature of each face is given below. Which will have the greatest positive power?
A)
10 cm convex and 15 concave
done
clear
B)
5 cm convex and 10 cm concave
done
clear
C)
15 cm convex and plane
done
clear
D)
20 cm convex and 30 cm concave
done
clear
View Answer play_arrow
question_answer 99) The slope of a graph drawn between threshold frequency and stopping potential is:
A)
e
done
clear
B)
h
done
clear
C)
\[\frac{h}{e}\]
done
clear
D)
he
done
clear
View Answer play_arrow
question_answer 100)
The truth table given below belongs for which gate? A B C 0 0 1 0 1 1 1 0 1 1 1 0
A)
OR
done
clear
B)
XOR
done
clear
C)
AND
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 101) Formalin is:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[HCOOH\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 102) Nesslers reagent is:
A)
\[NaHgC{{l}_{4}}\]
done
clear
B)
\[{{K}_{2}}Hg{{l}_{4}}\]
done
clear
C)
\[Hg{{(N{{H}_{3}})}_{2}}Cl\]
done
clear
D)
\[{{K}_{2}}Hf{{I}_{4}}+KOH\]
done
clear
View Answer play_arrow
question_answer 103) The process of zinc-plating on iron sheet is known as:
A)
aneling
done
clear
B)
roasting
done
clear
C)
galvanization
done
clear
D)
smelting
done
clear
View Answer play_arrow
question_answer 104) The correct one for \[d-\]orbital is:
A)
\[(n-1){{d}^{1-9}}n{{s}^{1}}\]
done
clear
B)
\[(n-1){{d}^{1-10}}n{{s}^{1-2}}\]
done
clear
C)
\[(n-1){{d}^{1-5}}\]
done
clear
D)
\[(n-1){{d}^{1-10}}n{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 105) The oxygen obtained from \[72\,\,kg\] of water is:
A)
\[72\,\,kg\]
done
clear
B)
\[46\,\,kg\]
done
clear
C)
\[50\,\,kg\]
done
clear
D)
\[64\,\,kg\]
done
clear
View Answer play_arrow
question_answer 106) The correct order of relative acidity is:
A)
\[HClO>HCl{{O}_{2}}>HCl{{O}_{3}}>HCl{{O}_{2}}\]
done
clear
B)
\[HCl{{O}_{4}}>HCl{{O}_{3}}>HCl{{O}_{2}}>HClO\]
done
clear
C)
\[HClO>HCl{{O}_{4}}>HCl{{O}_{2}}>HCl{{O}_{3}}\]
done
clear
D)
\[HCl{{O}_{3}}>HCl{{O}_{2}}>HCl{{O}_{4}}>HClO\]
done
clear
View Answer play_arrow
question_answer 107) The oxidation state of \[{{M}^{3+}}\] after removing three electrons is:
A)
\[zero\]
done
clear
B)
\[+3\]
done
clear
C)
\[+6\]
done
clear
D)
\[-6\]
done
clear
View Answer play_arrow
question_answer 108) The oil of wintergreen is:
A)
ethyl salicylate
done
clear
B)
methyl salicylate
done
clear
C)
benzaldehyde
done
clear
D)
phenyl salicylate
done
clear
View Answer play_arrow
question_answer 109) The lightest as is:
A)
\[{{N}_{2}}\]
done
clear
B)
\[Ar\]
done
clear
C)
\[Rn\]
done
clear
D)
\[He\]
done
clear
View Answer play_arrow
question_answer 110) The half-life period of radium is 1580 years. It remains 1/16 after the years:
A)
1580 years
done
clear
B)
3160 years
done
clear
C)
4740 years
done
clear
D)
6320 years
done
clear
View Answer play_arrow
question_answer 111) \[_{90}{{K}^{40}}\]and \[_{20}C{{a}^{40}}\] are known as:
A)
isotopes
done
clear
B)
isobars
done
clear
C)
inslones
done
clear
D)
isodiaphers
done
clear
View Answer play_arrow
question_answer 112) The ore of aluminium is:
A)
carnalite
done
clear
B)
malachite
done
clear
C)
galena
done
clear
D)
bauxite
done
clear
View Answer play_arrow
question_answer 113) The equivalent weight of \[KMn{{O}_{4}}\] in acidic medium is:
A)
\[158\]
done
clear
B)
\[52.67\]
done
clear
C)
\[31.6\]
done
clear
D)
\[49\]
done
clear
View Answer play_arrow
question_answer 114) The species responsible for nitraction is:
A)
\[NO_{2}^{+}\]
done
clear
B)
\[N{{O}_{3}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 115) A compound \[(60\,\,g)\] on analysis produce carbon, hydrogen and oxygen\[24\,\,g\], \[4\,\,g\] and \[32\,\,g\] respectively. The empirical formula is:
A)
\[{{C}_{2}}{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
C)
\[C{{H}_{2}}O\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 116) The indicator used for the filtration of weak base and strong acid is:
A)
thymol blue
done
clear
B)
methyl orange
done
clear
C)
phenolphthalein
done
clear
D)
fluorescein
done
clear
View Answer play_arrow
question_answer 117) The reagent used for Friedel Crafts reaction is:
A)
dry ether
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
anhydrous\[AlC{{l}_{3}}\]
done
clear
D)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 118) For converting a solution of \[100\,\,ml\,\,KCl\] of \[0.4\,\,M\] concentration into a solution of \[KCl\,\,0.05\,\,M\] concentration. The quantity of water added is:
A)
\[900\,\,ml\]
done
clear
B)
\[700\,\,ml\]
done
clear
C)
\[500\,\,ml\]
done
clear
D)
\[300\,\,ml\]
done
clear
View Answer play_arrow
question_answer 119) Buffer is a colloidal solution of:
A)
solid - solid
done
clear
B)
liquid - solid
done
clear
C)
solid - liquid
done
clear
D)
gas - solid
done
clear
View Answer play_arrow
question_answer 120) Acetone and chloroform reacts to produce:
A)
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
question_answer 121) The Claisen condensation reaction is given by:
A)
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
question_answer 122) The laboratory method for the preparation of\[{{H}_{2}}{{O}_{2}}\]is by:
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[N{{H}_{4}}HS{{O}_{4}}\]
done
clear
C)
\[N{{a}_{2}}{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 123) Picric acid is:
A)
trinitro toluene
done
clear
B)
trinitro benzene
done
clear
C)
trinitro phenol
done
clear
D)
trinitro aniline
done
clear
View Answer play_arrow
question_answer 124) \[_{90}T{{h}^{232}}{{\xrightarrow{{}}}_{82}}P{{b}^{208}}\] The number of \[\alpha \] and \[\beta \] particles emitted during the above reaction is:
A)
\[8\alpha \]and\[4\beta \]
done
clear
B)
\[8\alpha \]and\[16\beta \]
done
clear
C)
\[4\alpha \]and\[2\beta \]
done
clear
D)
\[6\alpha \]and\[4\beta \]
done
clear
View Answer play_arrow
question_answer 125) The phenomenon of mutation is:
A)
chemical change in DNA molecule
done
clear
B)
production of macromolecules
done
clear
C)
synthesis of macromolecules
done
clear
D)
invasion of foreign micro-organism
done
clear
View Answer play_arrow
question_answer 126) The \[cis\] and trails isomers are represented by:
A)
pent-1-ene
done
clear
B)
but-2-ene
done
clear
C)
prop-1-ene
done
clear
D)
but-1-ene
done
clear
View Answer play_arrow
question_answer 127) Duralumin is an alloy of:
A)
\[Al+Mn\]
done
clear
B)
\[Al+Mg+Ni+Mn\]
done
clear
C)
\[Al+Mg+Ni\]
done
clear
D)
\[Al+Mg+Mn+Cu\]
done
clear
View Answer play_arrow
question_answer 128) The incorrect statement for \[d-\]block element is:
A)
it shows magnetic property
done
clear
B)
it has variable valency
done
clear
C)
it has tendency for formation of coloured ions
done
clear
D)
it has complete \[d-\]orbitals
done
clear
View Answer play_arrow
question_answer 129) The hybridisation present in \[I{{F}_{3}}\] is:
A)
\[s{{p}^{3}}d\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
D)
\[s{{p}^{3}}{{d}^{3}}\]
done
clear
View Answer play_arrow
question_answer 130) The \[IUPAC\] name of \[\begin{align} & C{{H}_{2}}=CH-CH-C{{H}_{2}}-C{{H}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\overset{|}{\mathop{C}}\,{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}} \\ \end{align}\] is:
A)
3-proply pentene-1
done
clear
B)
3-ethyl-penten-1
done
clear
C)
4-ethyl-hexenc-1
done
clear
D)
3-ethyl-hexene-1
done
clear
View Answer play_arrow
question_answer 131) The relation of \[\Delta H\] and \[\Delta E\] is represented as:
A)
\[\Delta H=\Delta E-P\Delta V\]
done
clear
B)
\[\Delta E=\Delta H-P\Delta V\]
done
clear
C)
\[\Delta H=\Delta E+\Delta nRT\]
done
clear
D)
\[\Delta E=\Delta V+\Delta H\]
done
clear
View Answer play_arrow
question_answer 132) The number of a and K bonds present in\[CH\equiv C-C{{H}_{2}}-CH=C{{H}_{2}}\]is:
A)
\[10\sigma 3\pi \]
done
clear
B)
\[11\sigma 2\pi \]
done
clear
C)
\[9\sigma 3\pi \]
done
clear
D)
\[10\sigma 2\pi \]
done
clear
View Answer play_arrow
question_answer 133) The incorrect statement for \[14\,\,g\] of \[CO\] is:
A)
it occupies \[2.24\,\,litre\] at\[NTP\]
done
clear
B)
it corresponds to \[\frac{1}{2}\] mole of\[CO\]
done
clear
C)
it corresponds to same mole of \[CO\] and \[{{N}_{3}}\]
done
clear
D)
it corresponds to\[3.01\times {{10}^{23}}\]molecules of \[CO\]
done
clear
View Answer play_arrow
question_answer 134) \[N{{H}_{4}}Cl(s)\xrightarrow{{}}N{{H}_{3}}(g)+HCl(g)\] When the above reaction occurs, the entropy
A)
remains same
done
clear
B)
decrease
done
clear
C)
increases
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 135) Carbogen is a mixture of:
A)
\[C{{O}_{2}}+{{N}_{2}}\]
done
clear
B)
\[CO+{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}+{{O}_{2}}\]
done
clear
D)
\[C+{{H}_{2}}+{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 136) The carboxylic acid which reduces Tollens reagent is:
A)
\[HCOOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 137) The dissociation constant of two acids \[H{{A}_{1}}\] and \[H{{A}_{2}}\] are \[3.0\times {{10}^{-4}}\] and \[1.8\times {{10}^{-5}}\] respectively. The relative strengths of the acids is:
A)
\[1:16\]
done
clear
B)
\[1:4\]
done
clear
C)
\[4:1\]
done
clear
D)
\[16:1\]
done
clear
View Answer play_arrow
question_answer 138) The moderator used in nuclear reactors is:
A)
\[TEL\]
done
clear
B)
\[{{D}_{2}}O\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[R-O-R\]
done
clear
View Answer play_arrow
question_answer 139) One mole of \[C{{H}_{4}}\] contains:
A)
\[4\,\,g\] atoms of hydrogen
done
clear
B)
\[3.0\,\,g\] atoms of carbon
done
clear
C)
\[6.02\times {{10}^{23}}\] atomy of hydrogen
done
clear
D)
\[1.81\times {{10}^{23}}\] molecules of\[C{{H}^{4}}\]
done
clear
View Answer play_arrow
question_answer 140) The electron affinity of \[Be\] is similar to:
A)
\[He\]
done
clear
B)
\[B\]
done
clear
C)
\[Li\]
done
clear
D)
\[Na\]
done
clear
View Answer play_arrow
question_answer 141) The oxidation number of sulphur in\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\] is:
A)
\[+1\]
done
clear
B)
\[+2\]
done
clear
C)
\[+3\]
done
clear
D)
\[-3\]
done
clear
View Answer play_arrow
question_answer 142) The number of double bonds in gammexane is:
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 143) Benzaldehyde is converted to benzy alcohol by:
A)
Wurtz reaction
done
clear
B)
Cannizzaro reaction
done
clear
C)
Fittig reaction
done
clear
D)
Wurz Fittig reaction
done
clear
View Answer play_arrow
question_answer 144) Sindoor is represented by:
A)
\[Pb{{(N{{O}_{3}})}_{2}}\]
done
clear
B)
\[PbC{{O}_{3}}Pb{{(OH)}_{2}}\]
done
clear
C)
\[Pb{{(OH)}_{2}}4PbC{{O}_{3}}\]
done
clear
D)
\[P{{b}_{3}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 145) The most polar bond is:
A)
\[O-F\]
done
clear
B)
\[N-Cl\]
done
clear
C)
\[N-F\]
done
clear
D)
\[N-N\]
done
clear
View Answer play_arrow
question_answer 146) Aniline reacts with chloroform in presence of alcoholic \[KOH\] to produce bad smelling compound. The compound produced is :
A)
\[{{C}_{6}}{{H}_{5}}NC\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CN\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}NH{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 147) Bronze is a mixture of:
A)
\[Pb+Sn\]
done
clear
B)
\[Cu+Sn\]
done
clear
C)
\[Cu+Zn\]
done
clear
D)
\[Pb+Zn\]
done
clear
View Answer play_arrow
question_answer 148) Atom bomb is based on the principle of:
A)
nuclear fusion
done
clear
B)
nuclear fission
done
clear
C)
nuclear transformation
done
clear
D)
carbon dating
done
clear
View Answer play_arrow
question_answer 149) Glycerol on oxidation with bismuth nitrate produce:
A)
glyceric acid
done
clear
B)
glyoxalic acid
done
clear
C)
oxalic acid
done
clear
D)
meso oxalic acid
done
clear
View Answer play_arrow
question_answer 150) The osmotic pressure of \[5%\] solution of cane-sugar at \[{{150}^{o}}C\] is:
A)
\[3.47\,\,atm\]
done
clear
B)
\[5.07\,\,atm\]
done
clear
C)
\[2.09\,\,atm\]
done
clear
D)
\[4.03\,\,atm\]
done
clear
View Answer play_arrow
question_answer 151) Cadmium rods are used for which purpose?
A)
Emit electrons
done
clear
B)
Absorb neutrons
done
clear
C)
Emit neutrons
done
clear
D)
absorb electrons
done
clear
View Answer play_arrow
question_answer 152) Propionic acid and \[KOH\] reacts to produce which one of the following?
A)
Potassium propionate
done
clear
B)
Propyl alcohol
done
clear
C)
Propionaldehyde
done
clear
D)
Does not react
done
clear
View Answer play_arrow
question_answer 153) What is the effect of dilution on the equivalent conductance of strong electrolyte?
A)
Decrease on dilution
done
clear
B)
Remains unchanged
done
clear
C)
Increase on dilution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 154) \[35.4\,\,mL\] of \[HO\] is required for the neutralization of a solution containing 0.275 g of sodium hydroxide. The normality of hydrochloric acid is?
A)
\[0.97\,\,N\]
done
clear
B)
\[0.142\,\,N\]
done
clear
C)
\[0.194\,\,N\]
done
clear
D)
\[0.244\,\,N\]
done
clear
View Answer play_arrow
question_answer 155) When \[{{H}_{2}}S\] gas is passed in a metal sulphate solution in presence of\[N{{H}_{4}}OH\], a white precipitate is produced. The metal is identified as:
A)
\[Zn\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Pb\]
done
clear
D)
\[Hg\]
done
clear
View Answer play_arrow
question_answer 156) The value of amu is which of the following?
A)
\[1.57\times {{10}^{-24}}kg\]
done
clear
B)
\[1.66\times {{10}^{-124}}kg\]
done
clear
C)
\[1.99\times {{10}^{-124}}kg\]
done
clear
D)
\[1.66\times {{10}^{-27}}kg\]
done
clear
View Answer play_arrow
question_answer 157) The molecule having largest dipole moment among the following is:
A)
\[CH{{I}_{3}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 158) When calcium acetate is distilled, it will produce which of the following compound?
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 159) Which of the following electronic configuration represents noble gas?
A)
\[n{{s}^{2}}n{{p}^{6}}\]
done
clear
B)
\[n{{s}^{2}}n{{p}^{2}}\]
done
clear
C)
\[n{{s}^{2}}n{{p}^{4}}\]
done
clear
D)
\[n{{s}^{2}}n{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 160) Sodium pyrophosphate is represented by which of the following formula?
A)
\[N{{a}_{2}}{{P}_{2}}{{O}_{4}}\]
done
clear
B)
\[N{{a}_{4}}{{P}_{2}}{{O}_{5}}\]
done
clear
C)
\[N{{a}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[N{{a}_{2}}{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 161) Which one of the following can produce hydrogen when treated with metallic sodium?
A)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 162) Which one of the following has unit positive charge and 1 amu mass?
A)
Electron
done
clear
B)
Neutron
done
clear
C)
Proton
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 163) What is the co-ordination number of body centred cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 164) Which gas is evolved by the treatment of magnesium with very dilute solution of\[HN{{O}_{3}}?\]
A)
\[{{N}_{2}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 165) Which of the following compound showy aromatic properties?
A)
Valine
done
clear
B)
Leucine
done
clear
C)
Serine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 166) Which one of the following pair shows Buffers solution?
A)
\[NaCl+NaOH\]
done
clear
B)
\[C{{H}_{3}}COONa+C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{H}_{3}}COON{{H}_{4}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}+CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 167) \[PC{{l}_{3}}\] and cold water reacts to produce which of the following?
A)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
C)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 168) Which of the following converts carbonyl compounds into hydrocarbons?
A)
\[{{H}_{2}}/Pt\]
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[Zn\text{-}H/HCl\]
done
clear
View Answer play_arrow
question_answer 169) Which of the following chloride is water insoluble?
A)
\[HCl\]
done
clear
B)
\[AgCl\]
done
clear
C)
Both a and b
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 170) How many neutrons are present in tritium nucleus?
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 171) The total number of protons in \[10\,\,g\] of calcium carbonate is\[({{N}_{0}}=6.023\times {{10}^{23}})\]:
A)
\[3.01\times {{10}^{24}}\]
done
clear
B)
\[4.06\times {{10}^{24}}\]
done
clear
C)
\[2.01\times {{10}^{24}}\]
done
clear
D)
\[3.02\times {{10}^{24}}\]
done
clear
View Answer play_arrow
question_answer 172) By dissolving \[5\,\,g\] substance in \[50\,\,g\] of water, the decrease in freezing point is \[{{1.2}^{o}}C\]. The\[g\] molal depression is\[{{185}^{o}}C\]. The molecular weight of substance is:
A)
\[105.4\]
done
clear
B)
\[118.2\]
done
clear
C)
\[137.2\]
done
clear
D)
\[154.2\]
done
clear
View Answer play_arrow
question_answer 173) The high boiling point of water is due to which reason:
A)
co-ordinate bonding
done
clear
B)
covalent bond
done
clear
C)
electrostatic force of attraction
done
clear
D)
hydrogen bonding
done
clear
View Answer play_arrow
question_answer 174) Which is correct for an endothermic reaction?
A)
\[\Delta H\] is positive
done
clear
B)
\[\Delta H\]is negative
done
clear
C)
\[\Delta H\]is negative
done
clear
D)
\[\Delta H=\]zero
done
clear
View Answer play_arrow
question_answer 175) Acetonitriles on hydrolysis produce which of the following?
A)
Amide
done
clear
B)
Acid
done
clear
C)
Amines
done
clear
D)
Carbonyl compounds
done
clear
View Answer play_arrow
question_answer 176) The radius of hydrogen atom is\[0.53\,\,\overset{o}{\mathop{A}}\,\]. The radius of \[_{3}L{{i}^{+2}}\] is of:
A)
\[1.27\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.17\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.57\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.99\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 177) The purest form of coal is:
A)
peat
done
clear
B)
anthracite
done
clear
C)
bituminous
done
clear
D)
lignite
done
clear
View Answer play_arrow
question_answer 178) The correct sequence of hybridisation of methane, ethene and acetylene is:
A)
\[sp,\,\,s{{p}^{2}},\,\,s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}},\,\,s{{p}^{3}},\,\,sp\]
done
clear
C)
\[s{{p}^{3}},\,\,s{{p}^{2}},\,\,sp\]
done
clear
D)
\[s{{p}^{3}},\,\,sp,\,\,s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 179) Which phosphorus reacts with \[KOH\] solution to produce phosphene gas?
A)
White phosphorus
done
clear
B)
Red phosphorus
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 180) Sturated fatty acids are represented by which of the formula?
A)
\[{{C}_{n}}{{H}_{n}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{3n}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+1}}\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 181) \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow{Sm/HCl}{{C}_{6}}{{H}_{5}}X\] is identified as:
A)
\[NO\]
done
clear
B)
\[-N{{H}_{2}}\]
done
clear
C)
\[NHOH\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 182) Acetic acid and\[{{P}_{2}}{{O}_{5}}\], reacts to produce which of the following?
A)
Acetic anhydride
done
clear
B)
Acetaldehyde
done
clear
C)
Phosphoric acid
done
clear
D)
Acetone
done
clear
View Answer play_arrow
question_answer 183) The test for unsaturation is confirmed by the decolonization of which of the following?
A)
Iodine water
done
clear
B)
\[CuS{{O}_{4}}\] solution
done
clear
C)
Bromine water
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 184) Which of the following element shows maximum valency?
A)
Carbon
done
clear
B)
Barium
done
clear
C)
Nitrogen
done
clear
D)
Sulphur
done
clear
View Answer play_arrow
question_answer 185) The volume of oxygen necessary for the complete combusion of \[20\,\,litre\] of propane is:
A)
\[40\,\,litre\]
done
clear
B)
\[60\,\,litre\]
done
clear
C)
\[80\,\,litre\]
done
clear
D)
\[100\,\,litre\]
done
clear
View Answer play_arrow
question_answer 186) Which reaction is used for the preparation of acetophenone?
A)
Reimer-Tiemann reaction
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Friedel Crafts reaction
done
clear
D)
Cannizzaros reaction
done
clear
View Answer play_arrow
question_answer 187) \[0.005\,\,M\]acid solution has\[5pH\]. The percentage ionization of acid is:
A)
\[0.8%\]
done
clear
B)
\[0.6%\]
done
clear
C)
\[0.4%\]
done
clear
D)
\[0.2%\]
done
clear
View Answer play_arrow
question_answer 188) \[PVC\]polymer can be prepared which of the monomer?
A)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CH-Cl\]
done
clear
View Answer play_arrow
question_answer 189) \[C{{H}_{3}}COOH\]is weaker acid than\[{{H}_{2}}S{{O}_{4}}\]. It is due to
A)
more ionization
done
clear
B)
less ionization
done
clear
C)
covalent bond
done
clear
D)
electrovalent bond
done
clear
View Answer play_arrow
question_answer 190) The ortho and para hydrogen differ in respect of which of the following?
A)
In the molecular weight
done
clear
B)
In the nature of spin of protons
done
clear
C)
In the nature of spin of electrons
done
clear
D)
In the number of protons
done
clear
View Answer play_arrow
question_answer 191) Which of the following is correct according to adsorption isotherm?
A)
\[\frac{X}{m}\propto {{P}^{o}}\]
done
clear
B)
\[\frac{X}{m}\propto P\]
done
clear
C)
\[\frac{X}{m}\propto \frac{1}{{{p}^{1/n}}}\] all of these
done
clear
D)
done
clear
View Answer play_arrow
question_answer 192) \[_{84}R{{n}^{219}}\] is a member of actinium series. The other member of this series is:
A)
\[_{89}A{{c}^{225}}\]
done
clear
B)
\[^{90}T{{h}^{232}}\]
done
clear
C)
\[_{15}{{P}^{35}}\]
done
clear
D)
\[_{92}{{U}^{235}}\]
done
clear
View Answer play_arrow
question_answer 193) The compressibility factor of an ideal gas is:
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 194) Soaps can be classified as:
A)
carbohydrates
done
clear
B)
ether
done
clear
C)
salts of fatty acids
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 195) In a chemical reaction two reactants takes part. The rate of reaction is directly proportional to the concentration of one of them and inversely proportional to the concentration of the other. The order of reaction is:
A)
\[zero\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 196) A gaseous mixture contains \[56\,\,g\] of \[{{N}_{2}}44\,\,g\] of \[C{{O}_{2}}\] and \[16\,\,g\] of\[C{{H}_{4}}\]. The total pressure of mixture is \[720\,\,mm\] of\[Hg\]. The partial pressure of methane is:
A)
\[75\,\,atm\]
done
clear
B)
\[160\,\,atm\]
done
clear
C)
\[180\,\,atm\]
done
clear
D)
\[215\,\,atm\]
done
clear
View Answer play_arrow
question_answer 197) The metal used to recover copper from a solution of \[CuS{{O}_{4}}\] is:
A)
\[Fe\]
done
clear
B)
\[Hg\]
done
clear
C)
\[Na\]
done
clear
D)
\[Ag\]
done
clear
View Answer play_arrow
question_answer 198) Amino acids have poptide linkage which is:
A)
\[-CO-NH-\]
done
clear
B)
\[-C-N{{H}_{2}}\]
done
clear
C)
\[SO-NH-\]
done
clear
D)
\[-CO-N-\]
done
clear
View Answer play_arrow
question_answer 199) Phenacetin is used as:
A)
antipyretics
done
clear
B)
antiseptics
done
clear
C)
analgesic
done
clear
D)
antimalarials
done
clear
View Answer play_arrow
question_answer 200) Gravity separation process is used for the concentration of:
A)
calm-nine
done
clear
B)
haematite
done
clear
C)
chalcopyrite
done
clear
D)
bauxite
done
clear
View Answer play_arrow
question_answer 201) Structural unit of human kidney is :
A)
nephron
done
clear
B)
ureter
done
clear
C)
loop of Henie
done
clear
D)
Bowmans capsule
done
clear
View Answer play_arrow
question_answer 202) Heart of elephant is:
A)
aeurogonic
done
clear
B)
myogenic
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 203) Citliated epithelium is present in:
A)
trachea
done
clear
B)
ureter
done
clear
C)
intestine
done
clear
D)
nasal chamber
done
clear
View Answer play_arrow
question_answer 204) Deltoid ridge is present in:
A)
hurnerus
done
clear
B)
tibia-fibula
done
clear
C)
radio-ulna
done
clear
D)
femur
done
clear
View Answer play_arrow
question_answer 205) One haemoglobin carries how many molecules of \[{{O}_{2}}\]?
A)
4
done
clear
B)
2
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 206) In which era life was absent:
A)
Archeozoic
done
clear
B)
Paleozoic
done
clear
C)
Proterzoic
done
clear
D)
Azoic
done
clear
View Answer play_arrow
question_answer 207) Function of mitochondria is:
A)
excretion
done
clear
B)
respiration
done
clear
C)
digestion
done
clear
D)
excretion and respiration
done
clear
View Answer play_arrow
question_answer 208) Pulp cavity of teeth is lined by:
A)
odontoblast
done
clear
B)
chondroblast
done
clear
C)
osteoblast
done
clear
D)
amyloblast
done
clear
View Answer play_arrow
question_answer 209) Secretion of gastric juice is controlled by :
A)
gastrin
done
clear
B)
cholecystokinin
done
clear
C)
enterogastrin
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 210) Meissner corpuscles are responsible for:
A)
heat and present in skin
done
clear
B)
cold and present in skill
done
clear
C)
pressure and present in skin
done
clear
D)
pain and present in skin
done
clear
View Answer play_arrow
question_answer 211) Pelivic girdle of rabbit consists of :
A)
ilium, ischium and pubis
done
clear
B)
ilium, ischium and coracoid
done
clear
C)
coracoid, scapula and clavicle
done
clear
D)
ilium, coracoid and scapula
done
clear
View Answer play_arrow
question_answer 212) Acoustic spots in frog are present in :
A)
ossious labyrinth
done
clear
B)
carotid
done
clear
C)
membranous labyrinth
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 213) Difference between bone and cartilage is :
A)
Haversian canal
done
clear
B)
blood vessel
done
clear
C)
lymph vessel
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 214) Separation of amino acid and carboxylic groups is called :
A)
deamination
done
clear
B)
excretion
done
clear
C)
egestion
done
clear
D)
transamination
done
clear
View Answer play_arrow
question_answer 215) Net gain of ATP in prokaryotes from a molecule of glucose when oxidized is :
A)
40
done
clear
B)
38
done
clear
C)
36
done
clear
D)
34
done
clear
View Answer play_arrow
question_answer 216) Mouth part of mosquito is :
A)
sucking and piercing type
done
clear
B)
sponging type
done
clear
C)
biting and chewing type
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 217) Which of the following is bot a source of vitamin A?
A)
Carrot
done
clear
B)
Mango
done
clear
C)
Apple
done
clear
D)
Yeast
done
clear
View Answer play_arrow
question_answer 218) Parathormone is secreted during :
A)
increased blood calcium level
done
clear
B)
decreased blood calcium level
done
clear
C)
increased blood sugar level
done
clear
D)
decreased blood sugar level
done
clear
View Answer play_arrow
question_answer 219) Which of the following is purely motor cranial nerve?
A)
Olfactory
done
clear
B)
Optic
done
clear
C)
Abducens
done
clear
D)
Vagus
done
clear
View Answer play_arrow
question_answer 220) Minimum regeneration power is present in :
A)
nervous tissue
done
clear
B)
connective tissue
done
clear
C)
epithelial tissue
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 221) Largest smooth muscle is present in :
A)
leg
done
clear
B)
thigh
done
clear
C)
uterus of pregnant woman
done
clear
D)
urethra
done
clear
View Answer play_arrow
question_answer 222) Respiratory centre is present in :
A)
cerebellum
done
clear
B)
cerebrum
done
clear
C)
medulla obtongata
done
clear
D)
hypothalamus
done
clear
View Answer play_arrow
question_answer 223) Absorption of \[{{H}_{2}}O\] in DCT is controlled by:
A)
ADH
done
clear
B)
ACTH
done
clear
C)
LH
done
clear
D)
oxytocin
done
clear
View Answer play_arrow
question_answer 224) Alary muscle is associated with :
A)
heart and circulation
done
clear
B)
malpighian tubules and excretion
done
clear
C)
trachea and respiration
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 225) Organ of mastication in cockroach is :
A)
labrum
done
clear
B)
labium
done
clear
C)
mandibles
done
clear
D)
maxilla
done
clear
View Answer play_arrow
question_answer 226) Which of the following is an insect?
A)
Moth
done
clear
B)
Mites
done
clear
C)
Prawn
done
clear
D)
Scorpion
done
clear
View Answer play_arrow
question_answer 227) Complete metamorphosis is found in :
A)
house-fly and mosquito
done
clear
B)
house-fly and cockroach
done
clear
C)
mosquito and cockroach
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 228) Forest of nephridia are present in :
A)
pharyngeal region
done
clear
B)
clitellar region
done
clear
C)
anal region
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 229) Common feature in earthworm and cockroach is:
A)
cuticle (exoskeleton)
done
clear
B)
solid and ventral nerve cord
done
clear
C)
nephridia
done
clear
D)
Malpighian tubules
done
clear
View Answer play_arrow
question_answer 230) In Ascaris, 3rd moulting takes place in :
A)
intestine
done
clear
B)
lung
done
clear
C)
liver
done
clear
D)
egg
done
clear
View Answer play_arrow
question_answer 231) Pseudocoelom is the characteristic of:
A)
annelida
done
clear
B)
Echinodermata
done
clear
C)
mollusca
done
clear
D)
nematode
done
clear
View Answer play_arrow
question_answer 232) In Hydra and present:
A)
one testes and one ovary
done
clear
B)
one testes and-many ovary
done
clear
C)
many testes and many ovary
done
clear
D)
many testes and one ovary
done
clear
View Answer play_arrow
question_answer 233) Wisdom teeth in human is :
A)
\[{{\text{3}}^{\text{rd}}}\] molar and 4 in number
done
clear
B)
\[{{\text{3}}^{\text{rd}}}\]molar and 2 in number
done
clear
C)
\[{{2}^{\text{rd}}}\] molar and 2 in number
done
clear
D)
\[{{2}^{\text{rd}}}\] molar and 2 in number
done
clear
View Answer play_arrow
question_answer 234) Recapitulation theory was proposed by :
A)
E. Haeckel
done
clear
B)
Mendel
done
clear
C)
Hugo does Vries
done
clear
D)
Von Baer
done
clear
View Answer play_arrow
question_answer 235) Down syndrome in due to :
A)
chromosome no. increase in 21st pair autosome
done
clear
B)
chromosome no. decrease in 21st pair autosome
done
clear
C)
chromosome no. increase in 18th pair autosome
done
clear
D)
chromosome no. decrease in 18th pair autosome
done
clear
View Answer play_arrow
question_answer 236) Pseudocoelom developed from :
A)
embryonic mesoderm
done
clear
B)
archenteron
done
clear
C)
blastocoel
done
clear
D)
blaslopore lip
done
clear
View Answer play_arrow
question_answer 237) Larva of Sycon is :
A)
parenchymula
done
clear
B)
amphiblastula
done
clear
C)
redia
done
clear
D)
trochophore
done
clear
View Answer play_arrow
question_answer 238) Choanocyte is the characteristic feature of:
A)
sponges
done
clear
B)
nrthropods
done
clear
C)
annelida
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 239) Kala-azar is caused by;
A)
Trypanosoma cruzi
done
clear
B)
Leishmaniti donovani
done
clear
C)
Trypanosoma brucei
done
clear
D)
Trypanosoma gambiense
done
clear
View Answer play_arrow
question_answer 240) Which one of the following pairs is correctly matched?
A)
Aedes - plague
done
clear
B)
Anopheles - malaria
done
clear
C)
House fly - yellow fever
done
clear
D)
Body louse - typhoid
done
clear
View Answer play_arrow
question_answer 241) First evidence of ceremonial burial of dead body and belief in religion have been found with fossil of:
A)
Neanderthal
done
clear
B)
Cro-magnon
done
clear
C)
Homo-erectus
done
clear
D)
Homo habilis
done
clear
View Answer play_arrow
question_answer 242) Barr body is associated with :
A)
sex chromosome of female
done
clear
B)
sex chromosome of male
done
clear
C)
autosome of female
done
clear
D)
autosome of male
done
clear
View Answer play_arrow
question_answer 243) Darwins Book Origin of New Species by Natural Selection was published in :
A)
1809
done
clear
B)
1859
done
clear
C)
1957
done
clear
D)
1869
done
clear
View Answer play_arrow
question_answer 244) All stages of Plasmodium get digested in stomach of female Anopheles except:
A)
sporozoite
done
clear
B)
gamctocyle
done
clear
C)
erythrocyte
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 245) Which one of the following couple were suggested by Doctors to not have more than one child?
A)
\[R{{h}^{+}}\] male and \[R{{h}^{-}}\] female
done
clear
B)
\[R{{h}^{-}}\] male and \[R{{h}^{+}}\] female
done
clear
C)
\[R{{h}^{+}}\] male and \[R{{h}^{-}}\] female
done
clear
D)
\[R{{h}^{-}}\] male and \[R{{h}^{-}}\] female
done
clear
View Answer play_arrow
question_answer 246) Colour blindness is due to :
A)
recessive female chromosome
done
clear
B)
dominant female chromosome
done
clear
C)
recessive male chromosome
done
clear
D)
dominant male chromosome
done
clear
View Answer play_arrow
question_answer 247) According to one of the most accepted theory, the earth atmosphere before any life had originated consisted of \[{{H}_{2}}O,\,{{H}_{2}},\,N{{H}_{2}}\] and :
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 248) Abiogenesis is the :
A)
origin of life from non-living organism
done
clear
B)
origin of life from living organism
done
clear
C)
origin of viruses and microbes
done
clear
D)
spontaneous generation
done
clear
View Answer play_arrow
question_answer 249) Alleles are:
A)
alternate forms of a gene
done
clear
B)
homologous chromosome
done
clear
C)
pair of sex chromosome
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 250) Genetic recombination is due to :
A)
fertilization and meiosis
done
clear
B)
mitosia and meiosis
done
clear
C)
fertilization and mitosis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 251) Pyramid of energy is always :
A)
upright
done
clear
B)
downward
done
clear
C)
upright in pond and downward in forest
done
clear
D)
upright in forest and downward in pond
done
clear
View Answer play_arrow
question_answer 252) Peacock eats a snake and snake eats frog and frog eats insect while insect eats green plant, then position of peacock is :
A)
primary producer
done
clear
B)
secondary producer
done
clear
C)
decomposer
done
clear
D)
top at the apex of food pyramid
done
clear
View Answer play_arrow
question_answer 253) Source of energy in an ecosystem is :
A)
sun
done
clear
B)
ATP
done
clear
C)
sugar made by plant
done
clear
D)
green plant
done
clear
View Answer play_arrow
question_answer 254) Effect of pollution is first marked on :
A)
micro-organisms
done
clear
B)
green vegetation of an area
done
clear
C)
food crop
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 255) Legume plants are important for atmosphere because they :
A)
help in \[N{{O}_{2}}\] fixation
done
clear
B)
do not help in \[N{{O}_{2}}\] fixation
done
clear
C)
increase soil fertility
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 256) Pollution is not caused by :
A)
thermal power plant
done
clear
B)
aulomobile
done
clear
C)
radioactive power plant
done
clear
D)
hydroelectric power plant
done
clear
View Answer play_arrow
question_answer 257) endosperm of angiospermic plant is :
A)
triploid
done
clear
B)
dipioid
done
clear
C)
haploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 258) Taj Mahal marble is affected by:
A)
\[S{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{O}_{3}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 259) Sub-divisions in cell cycle are :
A)
\[S,\,{{G}_{2}},\,M\] and \[{{G}_{1}}\]
done
clear
B)
\[{{G}_{1}},\,{{G}_{2}}\] and M
done
clear
C)
\[S,\,M,\,{{G}_{1}}\] and \[{{G}_{2}}\]
done
clear
D)
\[{{G}_{2}},\,{{G}_{1}},\,M\] and S
done
clear
View Answer play_arrow
question_answer 260) Minimata disease first occured in :
A)
Japan
done
clear
B)
China
done
clear
C)
Korea
done
clear
D)
Russia
done
clear
View Answer play_arrow
question_answer 261) Sexual reproduction leads to :
A)
genetic recombination
done
clear
B)
polypoidy
done
clear
C)
aneuploidy
done
clear
D)
euploidy
done
clear
View Answer play_arrow
question_answer 262) 5th June is celebrated as :
A)
World forest day
done
clear
B)
World environment day
done
clear
C)
World Red Cross day
done
clear
D)
World food day
done
clear
View Answer play_arrow
question_answer 263) Which of the following soil is transported by air?
A)
Alluvial
done
clear
B)
Aerial
done
clear
C)
Eolian
done
clear
D)
Glacial
done
clear
View Answer play_arrow
question_answer 264) Plants growing in dry and saline soil are called :
A)
xerophyte
done
clear
B)
hydrophyte
done
clear
C)
halophyte
done
clear
D)
heliophyte
done
clear
View Answer play_arrow
question_answer 265) Which one of the following is well-developed present in hydropyte?
A)
Aerenchyma
done
clear
B)
Collenchyma
done
clear
C)
Stomata
done
clear
D)
Root system
done
clear
View Answer play_arrow
question_answer 266) Tropical dense forests are due to :
A)
low rainfall and low temperature
done
clear
B)
high rainfall and low temperature
done
clear
C)
low rainfall and high temperature
done
clear
D)
high rainfall and high temperature
done
clear
View Answer play_arrow
question_answer 267) Aquatic photo diffraction is :
A)
euphotic, disphotic and aphotic
done
clear
B)
aphotic, euphotic and disphotic
done
clear
C)
euphotic, aphotic and disphotic
done
clear
D)
euphotic, disphotic and aphotic
done
clear
View Answer play_arrow
question_answer 268) Community is:
A)
a group of independent and interacting population of different species
done
clear
B)
a group of independent and interacting population of same species
done
clear
C)
a group of independent and interacting population of same species in a specific area
done
clear
D)
a group of independent and interacting population of different species in a specific area
done
clear
View Answer play_arrow
question_answer 269) Terracing is done in :
A)
desert areas
done
clear
B)
hilly areas
done
clear
C)
dry areas
done
clear
D)
plain areas
done
clear
View Answer play_arrow
question_answer 270) Term ecosystem was given by :
A)
Odum
done
clear
B)
Koestler
done
clear
C)
Tansley
done
clear
D)
Mobius and Forbes
done
clear
View Answer play_arrow
question_answer 271) Step of respiration arc controlled by :
A)
substrates
done
clear
B)
enzymes
done
clear
C)
hormone
done
clear
D)
bile juice
done
clear
View Answer play_arrow
question_answer 272) Opening of flower is an example of:
A)
spontaneous movement
done
clear
B)
hyponastic movement
done
clear
C)
epinastic movement
done
clear
D)
cleistogamous movement
done
clear
View Answer play_arrow
question_answer 273) Removal of ring wood of tissue outside the vascular cambium from the tree trunk kills it because :
A)
water cannot move up
done
clear
B)
food does not travel down and root become starved
done
clear
C)
shoot become starved
done
clear
D)
annual ring and not produced
done
clear
View Answer play_arrow
question_answer 274) Law of Mendel which is not completely applicable is?
A)
Co-dominance
done
clear
B)
Law of segregation
done
clear
C)
Law of independent assortment
done
clear
D)
Law of dominance
done
clear
View Answer play_arrow
question_answer 275) Prokaryotic genetic system his :
A)
DNA and histone
done
clear
B)
DNA and no histone
done
clear
C)
no DNA and histone
done
clear
D)
no DNA and no histone
done
clear
View Answer play_arrow
question_answer 276) Study of environment and animal relation is :
A)
ecosystem
done
clear
B)
phytosociology
done
clear
C)
biotic community
done
clear
D)
ecology
done
clear
View Answer play_arrow
question_answer 277) A root was described as it adventitious root because:
A)
arose from plumule
done
clear
B)
was used variously for storage of food
done
clear
C)
was swollen
done
clear
D)
was growing in marshy place
done
clear
View Answer play_arrow
question_answer 278) Cycas revoluta is popularly known as :
A)
data palm
done
clear
B)
sago palm
done
clear
C)
sea palm
done
clear
D)
royal palm
done
clear
View Answer play_arrow
question_answer 279) Circinate vernation is present in :
A)
moss
done
clear
B)
fern
done
clear
C)
algae
done
clear
D)
gymnosperm
done
clear
View Answer play_arrow
question_answer 280) Wilting of plant is because:
A)
xylem is blocked
done
clear
B)
phloem is blocked
done
clear
C)
epidermis and few roots are removed
done
clear
D)
pith is removed
done
clear
View Answer play_arrow
question_answer 281) Auxanometer is used to detect:
A)
respiration
done
clear
B)
transpiration
done
clear
C)
plant movement
done
clear
D)
growth
done
clear
View Answer play_arrow
question_answer 282) Smallest plant which contain green pigment such as higher green plant is :
A)
schizomycetes
done
clear
B)
rhodophyceae
done
clear
C)
chlorophyceae
done
clear
D)
phaeophyceae
done
clear
View Answer play_arrow
question_answer 283) Term basal body is associated with the development of:
A)
cilia and flagella
done
clear
B)
cell plate
done
clear
C)
phragmoplast
done
clear
D)
kinetochore
done
clear
View Answer play_arrow
question_answer 284) Golgi body originates from :
A)
lysosome
done
clear
B)
endoplasmic reticulum
done
clear
C)
mitochondria
done
clear
D)
cell membrane
done
clear
View Answer play_arrow
question_answer 285) Lipid molecules in plasma membrane are arranged in which manner?
A)
scattered
done
clear
B)
series
done
clear
C)
alternate
done
clear
D)
head parallel
done
clear
View Answer play_arrow
question_answer 286) Structure of nuclear membrane helps in :
A)
organization of the spindle
done
clear
B)
synapsis of homologous chromosome
done
clear
C)
nucleo-cytoplasmic exchange of material
done
clear
D)
anaphasic sepration of daughter chromosome
done
clear
View Answer play_arrow
question_answer 287) Quantasomes are found in :
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
lysosome
done
clear
D)
endoplasmic retiuculum
done
clear
View Answer play_arrow
question_answer 288) Hydrolytic enzymes are stored in :
A)
Golgi bodies
done
clear
B)
Lysosumes
done
clear
C)
Endoplasmic reticulum
done
clear
D)
Mitochondria
done
clear
View Answer play_arrow
question_answer 289) Micronutrients are :
A)
an important as macro nutrient but are required in small amount
done
clear
B)
less important than macro nutrient
done
clear
C)
called micro as they play only a minor role in plant nutrition
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 290) Quiescent centre is present in :
A)
shoot apex
done
clear
B)
root apex
done
clear
C)
both a and b
done
clear
D)
meristematic tissue
done
clear
View Answer play_arrow
question_answer 291) Chlamydomonas occurs in:
A)
fresh water
done
clear
B)
ponds and lake
done
clear
C)
river
done
clear
D)
ocean
done
clear
View Answer play_arrow
question_answer 292) Ainsworths put Rhizopus in :
A)
zygomycotina
done
clear
B)
mastigomycotina
done
clear
C)
myxomycotina
done
clear
D)
ascomycotina
done
clear
View Answer play_arrow
question_answer 293) Root hair absorb water from the soil on account of:
A)
turgor pressure
done
clear
B)
osmotic pressure
done
clear
C)
suction pressure
done
clear
D)
root pressure
done
clear
View Answer play_arrow
question_answer 294) Smallest bacteria is :
A)
Spirillum
done
clear
B)
Bacillus
done
clear
C)
Dialister
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 295) During photosynthesis :
A)
\[{{O}_{2}}\] evolved comes from \[C{{O}_{2}}\]
done
clear
B)
ATP is formed
done
clear
C)
ATP is not formed
done
clear
D)
water is required as medium but it does not take part in photosynthesis
done
clear
View Answer play_arrow
question_answer 296) Mosses occurs in moist place because :
A)
they cannot grow on land
done
clear
B)
their gamete fuse in water
done
clear
C)
they lack vascular tissue
done
clear
D)
they lack root and stomata
done
clear
View Answer play_arrow
question_answer 297) Cuticle is secreted by :
A)
epidermis
done
clear
B)
endodermis
done
clear
C)
both (a) and (b)
done
clear
D)
hypodermis
done
clear
View Answer play_arrow
question_answer 298) Ribosome may also be called :
A)
Microsome
done
clear
B)
Dictyosome
done
clear
C)
Ribonucleo protein
done
clear
D)
Oxysomes
done
clear
View Answer play_arrow
question_answer 299) Plasmids occur in:
A)
Viruses
done
clear
B)
Chromosomes
done
clear
C)
Bacteria
done
clear
D)
Chloroplasts
done
clear
View Answer play_arrow
question_answer 300) A place was rocky and barren but now there is a green forest; the sequence of origin is:
A)
lichen, moss, herbs, shrubs
done
clear
B)
moss, lichen, herbs, shrubs
done
clear
C)
lichen, moss, shrubs, herbs
done
clear
D)
shrubs, herbs, moss, lichen
done
clear
View Answer play_arrow