question_answer 1) If M is the mass of the earth and R its radius, the ratio of the gravitational acceleration and the gravitational constant is:
A)
\[\frac{{{R}^{2}}}{M}\]
done
clear
B)
\[\frac{M}{{{R}^{2}}}\]
done
clear
C)
\[M{{R}^{2}}\]
done
clear
D)
\[\frac{M}{R}\]
done
clear
View Answer play_arrow
question_answer 2) A student unable to answer a question on Newtons laws of motion attempts to pull himself up by tugging on his hair. He will not succeed:
A)
as the force exerted is small
done
clear
B)
the frictional force while gripping, is small
done
clear
C)
Newtons law of inertia is not applicable to living beings
done
clear
D)
as the force applied is internal to the system
done
clear
View Answer play_arrow
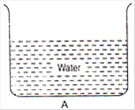
question_answer 3)
From the adjacent figure, the correct observation is:
A)
the pressure on the bottom of tank A is greater than at the bottom of B
done
clear
B)
the pressure on the bottom of the tank A is smaller than at the bottom of B
done
clear
C)
die pressure depends on the shape of the container
done
clear
D)
the pressure on the bottom of A and B is the same
done
clear
View Answer play_arrow
question_answer 4) Which one of the following is not a unit of youngs modulus?
A)
\[N{{m}^{-1}}\]
done
clear
B)
\[N{{m}^{-2}}\]
done
clear
C)
dyne \[c{{m}^{-2}}~~~~~~\]
done
clear
D)
mega pascal
done
clear
View Answer play_arrow
question_answer 5) A piece of blue glass heated to a high temperature and a piece of red glass at room temperature, are taken inside a dimly lit room, then:
A)
the blue piece will look blue and red will look as usual
done
clear
B)
red looks brighter red and blue looks ordinary blue
done
clear
C)
blue shines like brighter red compared to the red piece
done
clear
D)
both the pieces will look equally red
done
clear
View Answer play_arrow
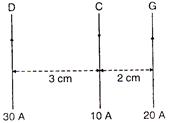
question_answer 6)
Three long, straight parallel wires, carrying current, are arranged as shown ill figure. The force experienced by a 25 cm length of wire C is:
A)
\[{{10}^{-3}}N~~~\]
done
clear
B)
\[2.5\times {{10}^{-3}}N\]
done
clear
C)
zero
done
clear
D)
\[1.5\times {{10}^{-3}}N\]
done
clear
View Answer play_arrow
question_answer 7) A 5.0 amp current is setup in an external circuit by a 6.0 volt storage battery for 6.0 minutes. The chemical energy of the battery is reduced by:
A)
\[1.08\times {{10}^{4}}J\]
done
clear
B)
\[1.08\times {{10}^{4}}J\]
done
clear
C)
\[1.8\times {{10}^{4}}J\]
done
clear
D)
\[1.8\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 8) The current in a simple series circuit is 5.0 amp. When an additional resistance of 2.0 ohms is inserted, the current drops to 4.0 amp. The original resistance of the circuit in ohms was:
A)
1.25
done
clear
B)
8
done
clear
C)
10
done
clear
D)
20
done
clear
View Answer play_arrow
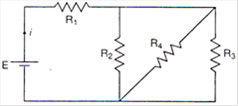
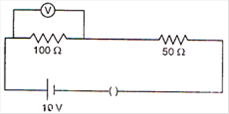
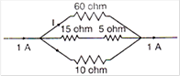
question_answer 9)
In the circuit given \[E=6.0\]volt, \[{{R}_{1}}=\text{ }100\,\Omega \]\[{{R}_{2}}=\text{ }{{R}_{3}}50\,\Omega \],\[{{R}_{4}}=75\,\Omega \]. The equivalent resistance of the circuit, in ohms, is:
A)
11.875
done
clear
B)
26.31
done
clear
C)
118.75
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 10) Two resistances are connected in two gaps of a metre bridge. The balance point is 20 cm from the zero end. A resistance of 15 ohms is connected in series with the smaller of the two. The null point shifts to 40 cm. The value of the smaller resistance in ohms is:
A)
3
done
clear
B)
6
done
clear
C)
9
done
clear
D)
12
done
clear
View Answer play_arrow
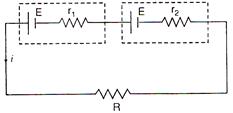
question_answer 11)
If the potential difference across the internal resistance r1 is equal to the emf E of the battery, then:
A)
\[R={{r}_{1}}+{{r}_{2}}\]
done
clear
B)
\[R=\frac{{{r}_{1}}}{{{r}_{2}}}\]
done
clear
C)
\[R={{r}_{1}}-{{r}_{2}}\]
done
clear
D)
\[R=\frac{{{r}_{2}}}{{{r}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 12) By using only two resistance coils-singly, in series or in parallel one should be able to obtain resistances of 3, 4, 12 and 16 ohms. The separate resistances of the coil are:
A)
3 and 4
done
clear
B)
4 and 12
done
clear
C)
12 and 16
done
clear
D)
16 and 3
done
clear
View Answer play_arrow
question_answer 13) The electrons in the beam of a television tube move horizontally from south to north. The vertical component of the earths magnetic field points down. The electron is deflected towards:
A)
west
done
clear
B)
no deflection
done
clear
C)
east
done
clear
D)
north to south
done
clear
View Answer play_arrow
question_answer 14) A tangent galvanometer has a reduction factor of 1 A arid it is placed with the plane of its coil perpendicular to die magnetic meridian. The deflection produced when a current of 1 A is passed through it is:
A)
\[60{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 15)
In the given circuit, the voltmeter records 5 volts. The resistance of the voltmeter in ohms is:
A)
200
done
clear
B)
100
done
clear
C)
10
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 16) The wavelength of the radiation emittec by a body depends upon:
A)
the nature of the surface
done
clear
B)
the area of the surface
done
clear
C)
the temperature of die surface
done
clear
D)
all of the above factors
done
clear
View Answer play_arrow
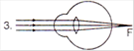
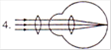
question_answer 17) Which mirror is to be used to obtain a parallel beam of light from a small lamp?
A)
Plane mirror
done
clear
B)
Convex mirror
done
clear
C)
Concave mirror
done
clear
D)
Any one of the above
done
clear
View Answer play_arrow
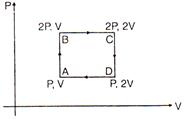
question_answer 18)
An ideal monoatomic gas is taken around the cycle ABCDA as shown in the PV diagram. The work done during the cycle is given by:
A)
\[\frac{1}{2}PV\]
done
clear
B)
\[PV\]
done
clear
C)
2 PV
done
clear
D)
4 PV
done
clear
View Answer play_arrow
question_answer 19) Which of the following is a wrong statement?
A)
D = 1/f where f is the focal length and D is called die refractive power of a lens.
done
clear
B)
Power is expressed in a diopter when f is in metres
done
clear
C)
Power is expressed in diopter and does not depend on the system of unit used to measure f
done
clear
D)
D is positive for convergent lens and negative for divergent lens.
done
clear
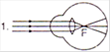
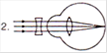
View Answer play_arrow
A)
1 represents far-sightedness
done
clear
B)
2 correction for short sightedness
done
clear
C)
3 represents far-sightedness
done
clear
D)
4 correction for far-sightedness
done
clear
View Answer play_arrow
question_answer 21) An electric field of 1500 V/m and a magnetic field of 0.40 weber/metre act on a moving electron. The minimum uniform speed along a straight line die electron could have is:
A)
\[1.6\times {{10}^{15}}m/s\]
done
clear
B)
\[6\times {{10}^{-16}}m/s\]
done
clear
C)
\[3.75\times {{10}^{3}}m/s\]
done
clear
D)
\[3.75\times {{10}^{2}}m/s\]
done
clear
View Answer play_arrow
question_answer 22) In an ammeter 10% of main current is passing through the galvanometer. If the resistance of the galvanometer is C, then the shunt resistance, in ohms is:
A)
9G
done
clear
B)
\[\frac{G}{9}\]
done
clear
C)
90G
done
clear
D)
\[\frac{G}{90}\]
done
clear
View Answer play_arrow
question_answer 23) Among the following properties describing diamagnetism identify the property that is wrongly stated:
A)
Diamagnetic material do not have permanent magnetic moment
done
clear
B)
Diamagnetism is explained in terms of electromagnetic induction
done
clear
C)
Diamagnetic materials have a small positive susceptibility
done
clear
D)
The magnetic moment of individual electrons neutralize each other
done
clear
View Answer play_arrow
question_answer 24) The induction coil works on the principle of:
A)
self-induction
done
clear
B)
mutual induction
done
clear
C)
Amperes rule
done
clear
D)
Flemings right hand rule
done
clear
View Answer play_arrow
question_answer 25) The square root of the product of inductance and capacitance has the dimension of:
A)
length
done
clear
B)
mass
done
clear
C)
time
done
clear
D)
no dimension
done
clear
View Answer play_arrow
A)
1 < 2 < 3
done
clear
B)
2 < 3 < 1
done
clear
C)
3 < 2 < 1
done
clear
D)
3 < 1 < 2
done
clear
View Answer play_arrow
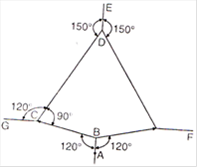
question_answer 27)
The adjacent figure is the part of a horizontally stretched net. Section AB is stretched with a force of 10 N. The tensions in the sections BC and BF are:
A)
10 N, 11 N
done
clear
B)
10 N, 6 N
done
clear
C)
10 N, 10 N
done
clear
D)
cant be calculated due to insufficient data
done
clear
View Answer play_arrow
question_answer 28) Out of the following four dimensional quantities, which one qualifies to be called a dimensional constant?
A)
Acceleration due to gravity
done
clear
B)
Surface tension of water
done
clear
C)
Weight of a standard kilogram mass
done
clear
D)
The velocity of light in vacuum
done
clear
View Answer play_arrow
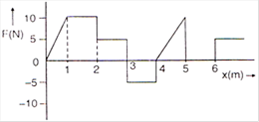
question_answer 29)
The relationship between the force F and position -v of a body is as shown in figure. The work done in displacing the body from x = 1 m to x = 5 m will be:
A)
30 J
done
clear
B)
15 J
done
clear
C)
25 J
done
clear
D)
20 J
done
clear
View Answer play_arrow
question_answer 30) From the top of a tower of two stones, whose masses are in the ratio 1:2 are thrown on straight up with an initial speed n and the second straight down with the same speed u. Then neglecting air resistance:
A)
the heavier stone hits die ground with a higher speed
done
clear
B)
the lighter stone hits the ground with a higher speed
done
clear
C)
both the stones will have the same speed when they hit the ground
done
clear
D)
the speed cant be determined with the given data
done
clear
View Answer play_arrow
question_answer 31) Infrared radiation was discovered in 1800 by:
A)
William Wollaston
done
clear
B)
William Herschel
done
clear
C)
Wilhelm Roentgen
done
clear
D)
Thomas Young
done
clear
View Answer play_arrow
question_answer 32) A particle on the trough of a wave at any instant will come to the mean position after a time (T = time period)
A)
T/2
done
clear
B)
T/4
done
clear
C)
T
done
clear
D)
2T
done
clear
View Answer play_arrow
question_answer 33) The disc of a siren containing 60 holes rotates at a constant speed of 360 rpm. The emitted sound is in unison with a tuning fork of frequency:
A)
10 Hz
done
clear
B)
360 Hz
done
clear
C)
216 Hz
done
clear
D)
60 Hz
done
clear
View Answer play_arrow
question_answer 34) The ratio of velocity of sound in hydrogen and oxygen at STP is:
A)
16 : 1
done
clear
B)
8 : 1
done
clear
C)
4 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 35) In an experiment with sonometer a tuning fork of frequency 256 Hz resonates with a length of 25 cm and another tuning fork resonates with a length of 16 cm. Tension of the string remaining constant the frequency of the second tuning fork is:
A)
163.84 Hz
done
clear
B)
400 Hz
done
clear
C)
320 Hz
done
clear
D)
204.8 Hz
done
clear
View Answer play_arrow
question_answer 36) The apparent frequency of a note is 200 Hz, when a listener is moving with a velocity of 40 ms-1 towards a stationary source. When he moves away from the same source with the same speed, the apparent frequency of the same note is 160 Hz. The velocity of sound in air in m/s is:
A)
340
done
clear
B)
330
done
clear
C)
360
done
clear
D)
320
done
clear
View Answer play_arrow
question_answer 37) The wave theory of light, in its original form, was first postulated by:
A)
Isaac Newton
done
clear
B)
Christian Huygens
done
clear
C)
Thomas Young
done
clear
D)
Augustin Jean Fresnel
done
clear
View Answer play_arrow
question_answer 38) If a liquid does not wet glass, its angle of contact is:
A)
zero
done
clear
B)
acute
done
clear
C)
obtuse
done
clear
D)
right angle
done
clear
View Answer play_arrow
question_answer 39)
The magnitude of I in ampere is:
A)
0.1
done
clear
B)
0.3
done
clear
C)
0.6
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 40) Electron of mass m arid charge q is travelling with a speed v along a circular path of radius r at right angles to a uniform magnetic field of intensity B. If the speed of the electron is doubled and the magnetic field is halved the resulting path would have a radius:
A)
2 r
done
clear
B)
4 r
done
clear
C)
r/4
done
clear
D)
r/2
done
clear
View Answer play_arrow
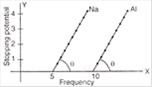
question_answer 41)
From the figure describing photoelectric effect we may infer correctly that:
A)
Na and Al both have the same threshold frequency
done
clear
B)
maximum kinetic energy for both the metals depend linearly on the frequency
done
clear
C)
the stopping potentials are different for Na and Al for the same change in frequency
done
clear
D)
Al is a better photo sensitive material than Na.
done
clear
View Answer play_arrow
question_answer 42) Two coherent light beams of intensity I and 4I are superposed. The maximum and minimum possible intensities in the resulting beam are:
A)
\[9I\]and \[I\]
done
clear
B)
\[9I\]and \[3I\]
done
clear
C)
\[5I\]and\[I\]
done
clear
D)
\[5I\]and \[3I\]
done
clear
View Answer play_arrow
question_answer 43) The electron in a hydrogen atom makes a transition from \[n={{n}_{1}}\]to \[n={{n}_{2}}\]state. The time period of the electron in the initial state \[\left( {{n}_{1}} \right)\] is eight times that in the final state\[\left( {{n}_{2}} \right)\]. The possible values of \[{{n}_{1}}\]and \[{{n}_{2}}\]are:
A)
\[{{n}_{1}}=8\],\[{{n}_{2}}=1\]
done
clear
B)
\[{{n}_{1}}=4\], \[{{n}_{2}}=2\]
done
clear
C)
\[{{n}_{1}}=2\],\[{{n}_{2}}=4\]
done
clear
D)
\[{{n}_{1}}=1\], \[{{n}_{2}}=8\]
done
clear
View Answer play_arrow
question_answer 44) If the forward voltage in a diode is increased, the width of the depletion region:
A)
increases
done
clear
B)
decreases
done
clear
C)
fluctuates
done
clear
D)
no change
done
clear
View Answer play_arrow
question_answer 45) Two nucleons are at a separation of one Fermi. Protons have a charge of\[+1.6\times {{10}^{-19}}C\]. The net nuclear force between them is\[{{F}_{1}}\], if both are neutrons, \[{{F}_{2}}\]if both are protons and \[{{F}_{3}}\] if one is proton and the other is neutron. Then:
A)
\[{{F}_{1}}=\text{ }{{F}_{2}}>\text{ }{{F}_{3}}\]
done
clear
B)
\[{{F}_{1}}={{F}_{2}}={{F}_{3}}\]
done
clear
C)
\[{{F}_{1}}<{{F}_{2}}<{{F}_{3}}\]
done
clear
D)
\[{{F}_{1}}>{{F}_{2}}>{{F}_{3}}\]
done
clear
View Answer play_arrow
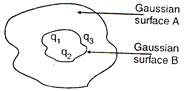
question_answer 46)
The electric flux for Gaussian surface A that enclose the charged particles in free space is (given \[\left( {{q}_{1}}=--14nC,{{q}_{2}}=78.85nC,{{q}_{3}}=-56nC \right)\]
A)
\[{{10}^{3}}N{{m}^{2~}}{{C}^{-1}}\]
done
clear
B)
\[{{10}^{3}}C{{N}^{-1}}{{m}^{-2}}\]
done
clear
C)
\[6.32\times {{10}^{3}}N{{m}^{2}}{{C}^{-1}}\]
done
clear
D)
\[6.32\times 103\text{ }C{{N}^{-1}}{{m}^{-2}}\]
done
clear
View Answer play_arrow
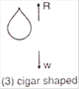
question_answer 47) Four metal conductors having different shapes 1. a sphere 2 cylinder 3. pear 4 lightning conductor are mounted on insulating stands and charged. The one which is best suited to retain the charges for a longer time is:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 48) The potential to which a conductor is raised, depends on:
A)
the amount of charge
done
clear
B)
geometry and size of the conductor
done
clear
C)
both [a] and [b]
done
clear
D)
only on [a]
done
clear
View Answer play_arrow
question_answer 49) The work done in carrying a charge q once round a circle of radius r with a charge Q at the centre is:
A)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}r}\]
done
clear
B)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}^{2}{{r}^{2}}}\]
done
clear
C)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}{{r}^{2}}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 50) An air filled parallel plate condenser has a capacity of\[2\text{ }pF\]. The separation of the plates is doubled and the interspace between the plates is filled with wax. If the capacity is increased to\[6\text{ }pF\], the dielectric constant of wax is:
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 51) The energy that should be added to an electron to reduce its de-Broglie wavelength from 1 nm to 0.5 is:
A)
four times the initial energy
done
clear
B)
equal to the initial energy
done
clear
C)
twice the initial energy
done
clear
D)
thrice the initial energy
done
clear
View Answer play_arrow
question_answer 52) Mean life of a radioactive sample is 100 seconds. Then its half-life (in minutes) is:
A)
0.693
done
clear
B)
1
done
clear
C)
\[{{10}^{-4}}\]
done
clear
D)
1.155
done
clear
View Answer play_arrow
question_answer 53) Consider two nuclei of the same radioactive nuclide. One of the nuclei was created in a supernova explosion 5 billion years ago. The other was created in a nuclear reactor 5 minutes ago. The probability of decay during the next time is:
A)
different for each nuclei
done
clear
B)
nuclei created in explosion decays first
done
clear
C)
nuclei created in the reactor decays first
done
clear
D)
independent of the time of creation
done
clear
View Answer play_arrow
question_answer 54) Bohrs atom model assumes:
A)
the nucleus is of infinite mass and is at rest
done
clear
B)
electrons in a quantized orbit will not radiate energy
done
clear
C)
mass of electron remains constant
done
clear
D)
all the above conditions
done
clear
View Answer play_arrow
question_answer 55) Identify the property which is not characteristic for a semiconductor?
A)
At a very low temperatures, it behaves like an insulator
done
clear
B)
At higher temperatures two types of charge carriers will cause conductivity
done
clear
C)
The charge carriers are electrons and holes in the valence band at higher temperatures
done
clear
D)
The semiconductor is electrically neutral.
done
clear
View Answer play_arrow
question_answer 56) Identify the wrong statement in the following. Coulombs law correctly described the electric force that:
A)
binds the electrons of an atom to its nucleus
done
clear
B)
binds the protons and neutrons in the nucleus of an atom
done
clear
C)
binds atoms together to form molecules
done
clear
D)
binds atoms and molecules to form solids
done
clear
View Answer play_arrow
question_answer 57) A single slit of width d is illuminated by violet light of wavelength 400 nm and the width of the diffraction pattern is measured as y. When half of the slit width is covered and illuminated by yellow light of wavelength 600 nm, the width of the diffraction pattern is:
A)
the pattern vanishes and the width is zero
done
clear
B)
\[y/3\]
done
clear
C)
\[3y\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 58) At Kavalur in India, the astronomers using a telescope whose objective had a diameter of one metre started using telescope of diameter 2.54 m. This resulted in:
A)
the increase in the resolving power by 2.54 times for the same A
done
clear
B)
the increase in the limiting angle by 2.54 times for the same X
done
clear
C)
decrease in the resolving power
done
clear
D)
no effect on the limiting angle
done
clear
View Answer play_arrow
question_answer 59) When unpolarised light beam is incident from air onto glass \[\left( n=1.5 \right)\] at the polarising angle:
A)
reflected beam is polarised 100 percent
done
clear
B)
reflected arid refracted beams are partially polarised
done
clear
C)
the reason for [a] is that almost all the light is reflected
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 60) Select the right option in the following:
A)
Christian Huygens, a contemporary of Newton established the wave theory of light by assuming that light waves were transverse.
done
clear
B)
Maxwell provided the theoretical evidence that light is transverse wave
done
clear
C)
Thomas Young experimentally proved the wave behavior of light and Huygens assumption
done
clear
D)
All the statements given above, correctly answers the question what is light?
done
clear
View Answer play_arrow
question_answer 61) 15 moles of \[{{H}_{2}}\]and 5.2 moles of \[{{I}_{2}}\] are mixed and allowed to attain equilibrium at \[{{500}^{o}}C\]. At equilibrium, the concentration of \[HI\] is found to be 10 moles. The equilibrium constant for the formation of \[HI\] is:
A)
\[50\]
done
clear
B)
\[15\]
done
clear
C)
\[100\]
done
clear
D)
\[25\]
done
clear
View Answer play_arrow
question_answer 62) If, in the reaction \[{{N}_{2}}{{O}_{4}}2N{{O}_{2}}\], x is, that part of \[{{N}_{2}}{{O}_{4}}\] which dissociates, then the number of molecules at equilibrium will be:
A)
\[1\]
done
clear
B)
\[3\]
done
clear
C)
\[(1+x)\]
done
clear
D)
\[{{(1+x)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 63) Which of these does not influence the rate of reaction?
A)
Nature of the reactants
done
clear
B)
Concentration of the reactants
done
clear
C)
Temperature of the reaction
done
clear
D)
Molecularity of the reaction
done
clear
View Answer play_arrow
question_answer 64) For the reaction \[A+B\xrightarrow{{}}C\], it is found that doubling the concentration of A increases the rate by 4 times, and doubling the concentration of B doubles the reaction rate. What is the overall order of the reaction?
A)
\[4\]
done
clear
B)
\[3/2\]
done
clear
C)
\[3\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 65) The rate at which a substance reacts depends on its:
A)
atomic weight
done
clear
B)
atomic number
done
clear
C)
molecular weight
done
clear
D)
active mass
done
clear
View Answer play_arrow
question_answer 66) A compound A has a molecular formula \[{{C}_{2}}C{{I}_{3}}OH\]. It reduces Fehlings solution and on oxidation, gives a monocarboxylic acid B. A can be obtained by the action of chlorine on ethyl alcohol. A is:
A)
chloroform
done
clear
B)
chloral
done
clear
C)
methyl chloride
done
clear
D)
monochloroacetic acid
done
clear
View Answer play_arrow
question_answer 67) Which of the following halo alkanes is most reactive?
A)
1-chloropropane
done
clear
B)
1-bromopropane
done
clear
C)
2-chloropropane
done
clear
D)
2-bromopropane
done
clear
View Answer play_arrow
question_answer 68) The reaction in which phenol differs from alcohol is:
A)
it undergoes esterification with carboxylic acid
done
clear
B)
it reacts with ammonia
done
clear
C)
it forms yellow crystals of iodoform
done
clear
D)
it liberates \[{{H}_{2}}\] with Na metal.
done
clear
View Answer play_arrow
question_answer 69) An organic compound A containing C, H and 0 has a pleasant odour with K-CET Medical Entrance Examination boiling point of\[{{78}^{o}}C\]. On boiling A with concentrated \[{{H}_{2}}S{{O}_{4}}\], a colourless gas is produced which decolourises bromine water and alkaline \[KMn{{O}_{4}}\]. The organic liquid A is:
A)
\[{{C}_{2}}{{H}_{5}}Cl\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}COOC{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 70) Which of the following is an amphoteric acid?
A)
Glycine
done
clear
B)
Salicylic acid
done
clear
C)
Benzoic acid
done
clear
D)
Citric acid
done
clear
View Answer play_arrow
question_answer 71) Gold is extracted by hydrometallurgical process, based on its property:
A)
of being electropositive
done
clear
B)
of being less reactive
done
clear
C)
to form complexes which are water soluble
done
clear
D)
to form salts which are water soluble
done
clear
View Answer play_arrow
question_answer 72) In blast furnace, iron oxide is reduced by:
A)
hot blast of air
done
clear
B)
carbon monoxide
done
clear
C)
carbon
done
clear
D)
silica
done
clear
View Answer play_arrow
question_answer 73) Which of the following pairs of elements cannot form an alloy?
A)
\[Zn,Cu\]
done
clear
B)
\[Fe,Hg\]
done
clear
C)
\[Fe,C\]
done
clear
D)
\[Hg,Na\]
done
clear
View Answer play_arrow
question_answer 74) Which compound is zero valent metal complex?
A)
\[[Cu{{(N{{H}_{3}})}_{4}}]S{{o}_{4}}\]
done
clear
B)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
C)
\[[Ni{{(CO)}_{4}}]\]
done
clear
D)
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
View Answer play_arrow
question_answer 75) Alum is a water purifier because it :
A)
coagulates the impurities
done
clear
B)
softens hard water
done
clear
C)
gives taste
done
clear
D)
destroys the pathogenic bacteria
done
clear
View Answer play_arrow
question_answer 76) For the reaction \[{{N}_{2}}_{(g)}+{{O}_{2}}_{(g)}2N{{O}_{(g)}}\]the value of \[{{K}_{c}}\] at \[{{800}^{o}}C\]is 0.1. When the equilibrium concentration of both the reactants is \[0.5mol\], what is the value of \[{{K}_{p}}\] at the same temperature?
A)
\[0.5\]
done
clear
B)
\[0.1\]
done
clear
C)
\[0.01\]
done
clear
D)
\[0.025\]
done
clear
View Answer play_arrow
question_answer 77) The extent of adsorption of a gas on a solid depends on:
A)
Nature of the gas
done
clear
B)
Pressure of the gas
done
clear
C)
Temperature of the gas
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 78) A emulsifier is a substance which:
A)
stabilises the emulsion
done
clear
B)
homogenises the emulsion
done
clear
C)
coagulates the emulsion
done
clear
D)
accelerates the dispersion of liquid in liquid
done
clear
View Answer play_arrow
question_answer 79) Which of the following types of metals form the most efficient catalysts?
A)
Alkali metals
done
clear
B)
Alkaline earth metals
done
clear
C)
Transition metals
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 80) The species among the following, which can act as an acid and a base is:
A)
\[HSO_{4}^{-}\]
done
clear
B)
\[SO_{4}^{2-}\]
done
clear
C)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 81) Benzyl alcohol and sodium benzoate is obtained by the action of sodium hydroxide on benzaldehyde. This reaction is known as:
A)
Perkins reaction
done
clear
B)
Cannizaros reaction
done
clear
C)
Sandmeyers reaction
done
clear
D)
Claisen condensation
done
clear
View Answer play_arrow
question_answer 82) Ethyl chloride on heating with \[AgCN\] forms a compound X. The functional isomer of X is:
A)
\[{{C}_{2}}{{H}_{5}}NC\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}CN\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 83) A compound, containing only carbon, hydrogen and oxygen, has a molecular weight of 44. On complete oxidation it is converted into a compound of molecular weight 60. The original compound is:
A)
an aldehyde
done
clear
B)
an acid
done
clear
C)
an alcohol
done
clear
D)
an ether
done
clear
View Answer play_arrow
question_answer 84) Grignard reagent adds to:
A)
\[>C=O\]
done
clear
B)
\[-C\equiv O\]
done
clear
C)
\[>C=S\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 85) Which of the following biomolecules contain non-transition metal ion?
A)
Vitamin \[{{B}_{12}}\]
done
clear
B)
Chlorophyll
done
clear
C)
Haemoglobin
done
clear
D)
Insulin
done
clear
View Answer play_arrow
question_answer 86) A mixture of two moles of carbon monoxide and one mole of oxygen, in a closed vessel is ignited to convert the carbon monoxide to carbon dioxide. If \[\Delta H\] is the enthalpy change and \[\Delta E\]is the change in internal energy, then:
A)
\[\Delta H>\Delta E\]
done
clear
B)
\[\Delta H<\Delta E\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
The relationship depends on the capacity of the vessel
done
clear
View Answer play_arrow
question_answer 87) The cooling in refrigerator is due to:
A)
Reaction of the refrigerator gas
done
clear
B)
Expansion of ice
done
clear
C)
The expansion of the gas in the refrigerator
done
clear
D)
The work of the compressor
done
clear
View Answer play_arrow
question_answer 88) For a system in equilibrium, \[\Delta G=0\], under conditions of constant .........
A)
Temperature and pressure
done
clear
B)
Temperature and volume
done
clear
C)
Pressure and volume
done
clear
D)
Energy and volume
done
clear
View Answer play_arrow
question_answer 89) Molar heat of vaporisation of a liquid is \[6kj\,mo{{l}^{-1}}\] . If the entropy change is \[16J\,mo{{l}^{-1}}{{K}^{-1}}\] , the boiling point of the liquid is :
A)
\[{{375}^{o}}C\]
done
clear
B)
\[375K\]
done
clear
C)
\[273K\]
done
clear
D)
\[{{102}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 90) The temperature of the system decreases in an:
A)
Adiabatic compression
done
clear
B)
Isothermal compression
done
clear
C)
Isothermal expansion
done
clear
D)
Adiabatic expansion
done
clear
View Answer play_arrow
question_answer 91) A buffer solution has equal volumes of\[0.2M\text{ }N{{H}_{4}}OH\]and \[0.02M\,N{{H}_{4}}Cl\]The \[_{p}{{k}_{a}}\] of the base is 5. The pH is:
A)
\[10\]
done
clear
B)
\[9\]
done
clear
C)
\[4\]
done
clear
D)
\[7\]
done
clear
View Answer play_arrow
question_answer 92) The hydrogen electrode is dipped in a solution of pH 3 at\[{{25}^{o}}C\]. The potential would be (the value of \[2.303\text{ }RT/F\]is \[0.059V\]):
A)
\[0.177V\]
done
clear
B)
\[0.087V\]
done
clear
C)
\[0.059V\]
done
clear
D)
\[-0.177V\]
done
clear
View Answer play_arrow
question_answer 93) \[20mL\] of \[0.5N\,HCl\]and \[35mL\]of \[0.1N\] \[NaOH\] are mixed. The resulting solution will:
A)
Be neutral
done
clear
B)
Be basic
done
clear
C)
Turn phenolphthalein solution pink
done
clear
D)
Turn methyl orange red
done
clear
View Answer play_arrow
question_answer 94) Corrosion of iron is essentially an electrochemical phenomenon where the cell reactions are:
A)
\[Fe\] is oxidised to \[F{{e}^{2+}}\] and dissolved oxygen in water is reduced to \[O{{H}^{-}}\]
done
clear
B)
\[Fe\] is oxidised to \[F{{e}^{3+}}\] and \[{{H}_{2}}O\] is reduced to \[O_{2}^{2-}\]
done
clear
C)
\[Fe\] is oxidised to \[F{{e}^{2+}}\] and \[{{H}_{2}}O\] is reduced to \[O_{2}^{-}\]
done
clear
D)
\[Fe\] is oxidised to \[F{{e}^{2+}}\] and \[{{H}_{2}}O\] is reduced to \[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 95) The standard electrode potential is measured by:
A)
Electrometer
done
clear
B)
Voltmeter
done
clear
C)
Pyrometer
done
clear
D)
Galvanometer
done
clear
View Answer play_arrow
question_answer 96) A precipitate of \[AgCl\] is formed when equal volumes of the following are mixed: [\[{{K}_{sp}}\] for \[AgCl={{10}^{-10}}\]]
A)
\[{{10}^{-4}}MAgN{{O}_{3}}\] And \[{{10}^{-7}}M\,\,HCl\]
done
clear
B)
\[{{10}^{-5}}M\,AgN{{O}_{3}}\] And \[{{10}^{-6}}M\,HCl\]
done
clear
C)
\[{{10}^{-5}}M\,AgN{{O}_{3}}\] And \[{{10}^{-4}}M\,HCl\]
done
clear
D)
\[{{10}^{-6}}M\,AgN{{O}_{3}}\] And \[{{10}^{-6}}M\,HCl\]
done
clear
View Answer play_arrow
question_answer 97) Which one of the following defects in the crystals lowers its density?
A)
Frenkel defect
done
clear
B)
Schottky defect
done
clear
C)
F-centres
done
clear
D)
Interstitial defect
done
clear
View Answer play_arrow
question_answer 98) A radioactive isotope has a half-life of 10 days. If today 125 mg is left over, what was its original weight 40 days earlier?
A)
\[2g\]
done
clear
B)
\[600mg\]
done
clear
C)
\[1g\]
done
clear
D)
\[1.5g\]
done
clear
View Answer play_arrow
question_answer 99) Which of the following cannot be accelerated?
A)
\[\alpha \]-particle
done
clear
B)
\[\beta \]-particle
done
clear
C)
Protons
done
clear
D)
Neutrons
done
clear
View Answer play_arrow
question_answer 100) In which of the following nuclear reaction neutrons is emitted?
A)
\[_{13}^{27}Al+_{2}^{4}He\to _{15}^{30}P\]
done
clear
B)
\[_{6}^{12}C+_{1}^{1}He\to _{7}^{13}N\]
done
clear
C)
(c} \[_{15}^{30}P\to _{14}^{30}Si\]
done
clear
D)
\[_{96}^{241}Am+_{2}^{4}He\to _{97}^{245}Bk\]
done
clear
View Answer play_arrow
question_answer 101) Molarity of \[0.2N\text{ }{{H}_{2}}S{{O}_{4}}\]is:
A)
\[0.2\]
done
clear
B)
\[0.4\]
done
clear
C)
\[0.6\]
done
clear
D)
\[0.1\]
done
clear
View Answer play_arrow
question_answer 102) In the equation of state of an ideal gas\[PV=nRT\], the value of the universal gas constant would depend only on:
A)
The nature of the gas
done
clear
B)
The pressure of the gas
done
clear
C)
The units of the measurement
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 103) A commercial sample of hydrogen peroxide is labelled as 10 volume. Its percentage strength is nearly:
A)
\[1%\]
done
clear
B)
\[3%\]
done
clear
C)
\[10%\]
done
clear
D)
\[90%\]
done
clear
View Answer play_arrow
question_answer 104) Activated charcoal is used to remove colouring matter from pure substances. It works by:
A)
Oxidation
done
clear
B)
Reduction
done
clear
C)
Bleaching
done
clear
D)
Adsorption
done
clear
View Answer play_arrow
question_answer 105) When plants and animals decay, the organic nitrogen is converted into inorganic nitrogen. The inorganic nitrogen is in the form of:
A)
Ammonia
done
clear
B)
Elements of nitrogen
done
clear
C)
Nitrates
done
clear
D)
Nitrides
done
clear
View Answer play_arrow
question_answer 106) Three dimensional molecules with cross links are formed in the case of a:
A)
thermoplastic
done
clear
B)
thermosetting plastic
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 107) Sucrose molecule is made up of:
A)
A gluco pyranose and a fructo pyranose
done
clear
B)
A gluco pyranose and a fructo furanose
done
clear
C)
A gluco furanose and a fructo pyranose
done
clear
D)
A gluco furanose and a fructo furanose
done
clear
View Answer play_arrow
question_answer 108) Water insoluble component of starch is:
A)
Amylopectin
done
clear
B)
Amylose
done
clear
C)
Cellulose
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 109) An example for a saturated fatty acid, present in nature is:
A)
Oleic acid
done
clear
B)
linoleic acid
done
clear
C)
linolenic acid
done
clear
D)
palmitic acid
done
clear
View Answer play_arrow
question_answer 110) A nanopeptide contains......peptide linkages:
A)
\[10\]
done
clear
B)
\[8\]
done
clear
C)
\[9\]
done
clear
D)
\[18\]
done
clear
View Answer play_arrow
question_answer 111) A gas decolourised by \[KMn{{O}_{4}}\] solution but gives no precipitate with ammoniacal cuprous chloride is:
A)
Ethane
done
clear
B)
Methane
done
clear
C)
ethane
done
clear
D)
Acetylene
done
clear
View Answer play_arrow
question_answer 112) \[{{H}_{3}}C-\underset{Cl}{\mathop{\underset{|}{\mathop{C}}\,}}\,=CH-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\]
A)
2-chloro-4-methyl-2pentene
done
clear
B)
4-chloro-2-methyl-3-pentene
done
clear
C)
4-methyl-2-chloro-2-pentene
done
clear
D)
2-chloro-4,4-dimethyl-2-butene
done
clear
View Answer play_arrow
question_answer 113) Amongst the following the compound that can most readily get sulphonated is:
A)
Benzene
done
clear
B)
Toluene
done
clear
C)
Nitrobenzene
done
clear
D)
Chlorobenzene
done
clear
View Answer play_arrow
question_answer 114) Household gaseous fuel (LPG) mainly contains:
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{4}}{{H}_{10}}\]
done
clear
View Answer play_arrow
question_answer 115) Use of chlorofluoro carbons is not encouraged because:
A)
They are harmful to the eyes of people that use it
done
clear
B)
They damage the refrigerators and air conditioners
done
clear
C)
They eat away the ozone in the atmosphere
done
clear
D)
They destroy the oxygen layer
done
clear
View Answer play_arrow
question_answer 116) An example of a sulphur containing amino acid is:
A)
Lysine
done
clear
B)
serine
done
clear
C)
Cysteine
done
clear
D)
tyrosine
done
clear
View Answer play_arrow
question_answer 117) Which of the following is not present in a nucleotide?
A)
Cytosine
done
clear
B)
Guanine
done
clear
C)
Adenine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 118) Antiseptic chloroxylenol is:
A)
4-chloro-3,5-dimethyl phenol
done
clear
B)
3-chloro-4,5-dimethyl phenol
done
clear
C)
4-chloro-2,5-dimethyl phenol
done
clear
D)
5-chJoro-3,4-dimethyl phenol
done
clear
View Answer play_arrow
question_answer 119) An atom of an element A has three electrons in its outermost orbit and that of B has six electrons in its outermost orbit. The formula of the compound between these two will be:
A)
\[{{A}_{3}}{{B}_{6}}\]
done
clear
B)
\[{{A}_{2}}{{B}_{3}}\]
done
clear
C)
\[{{A}_{3}}{{B}_{2}}\]
done
clear
D)
\[{{A}_{2}}B\]
done
clear
View Answer play_arrow
question_answer 120) Among \[N{{a}^{+}},Na,Mg\] and \[M{{g}^{2+}}\], the largest particle is:
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Na\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 121) Identify from the following, a characteristic pigment which contain copper containing protein:
A)
plastoquinone
done
clear
B)
ferredoxin
done
clear
C)
cytochrome
done
clear
D)
plastocyanin
done
clear
View Answer play_arrow
question_answer 122) In which of the following regions of a nephron does maximum reabsorption of useful substances, takes place?
A)
Henles loop
done
clear
B)
Glomerulus
done
clear
C)
Proximal convoluted tubule
done
clear
D)
Distal convoluted tubule
done
clear
View Answer play_arrow
question_answer 123) Which of the following statements is true with reference to cross pollination in angiosperms?
A)
It requires the production of a large number of pollen grains
done
clear
B)
It can fail to occur due to distance barrier
done
clear
C)
It occurs only in unisexual flowers
done
clear
D)
It most often results in high yield of plants
done
clear
View Answer play_arrow
question_answer 124) Which of the following natural process is likely to hasten organic evolution?
A)
Favourable environment
done
clear
B)
Overproduction
done
clear
C)
Abundant genotypic variations
done
clear
D)
Reproductive isolation
done
clear
View Answer play_arrow
question_answer 125) A technology which has found immense use in solving cases of disputed parentage, is:
A)
polyniorasc chain reaction
done
clear
B)
DNA finger printing
done
clear
C)
monoclonal antibody production
done
clear
D)
recombinant DNA technology
done
clear
View Answer play_arrow
question_answer 126) Green house effect is the cumulative result of the influences of certain gases. Identify the gas, which is not involved in this influence:
A)
methane
done
clear
B)
chlorofluorocarbons
done
clear
C)
nitrogen
done
clear
D)
carbon dioxide
done
clear
View Answer play_arrow
question_answer 127)
Column - I lists some principles, pertaining to physiology of plants. Column - II lists the names of scientist who proposed the idea. Match the two columns. Identify the correct choice from the given: Column-I Column-II A. Mass flow hypothesis p. J. C. Bose B. Relay pump theory q. Strasburger C. Transpiration pull theory r. Munch D. Pulsatile movement theory s. Godlewski t. Dixon and Jolly
A)
A = r; B = s; C = t; D = p
done
clear
B)
A = r; B = s; C = p; D = t
done
clear
C)
A = s; B = r; C = p; D = t
done
clear
D)
A = s; B = r; C = t; D = p
done
clear
View Answer play_arrow
question_answer 128) Which one of the following types of silk is being produced extensively in South East Asia:
A)
Eri
done
clear
B)
Mulberry
done
clear
C)
Tassah
done
clear
D)
Muga
done
clear
View Answer play_arrow
question_answer 129) Identify from the following plant parts, the major contributors to human food?
A)
Stem
done
clear
B)
Root
done
clear
C)
Fruits
done
clear
D)
Leaves
done
clear
View Answer play_arrow
question_answer 130) Alcohol is the most socially accepted narcotic drug. Excessive consumption of alcohol leads to:
A)
state of hallucination
done
clear
B)
loss of memory
done
clear
C)
suppression of brain function
done
clear
D)
cirrhosis of liver
done
clear
View Answer play_arrow
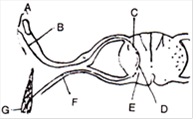
question_answer 131)
The following figure shows the stomatal apparatus. Identify the parts labelled as A, B, C and D. Choose the correct answer from the following:
A)
A = Guard cells, B = Stoma, C = Chloroplasts, D = Subsidiary cells
done
clear
B)
A = Subsidiary cells, B = Chloroplasts, C = Stoma, D = Guard cells
done
clear
C)
A = Guard cells, B = Chloroplasts, C = Stoma, D = Subsidiary cells
done
clear
D)
A = Subsidiary cells, B = Stoma, C = Chloroplasts, D = Guard cells
done
clear
View Answer play_arrow
question_answer 132) In which of the following plants, there will be 110 transpiration?
A)
Aquatic, submerged plants
done
clear
B)
Plants living in deserts
done
clear
C)
Aquatic plants with floating leaves
done
clear
D)
Plants growing in hilly regions
done
clear
View Answer play_arrow
question_answer 133) Which of the following groups of algae do not have eukaryotic organization?
A)
Green algae
done
clear
B)
Blue - green algae
done
clear
C)
Red algae
done
clear
D)
Golden brown algae
done
clear
View Answer play_arrow
question_answer 134) Identify from the following a hormone produced by the pituitary gland in both males and females but functional only in females:
A)
vasopressin
done
clear
B)
relaxin
done
clear
C)
prolactin
done
clear
D)
somatotropic hormone
done
clear
View Answer play_arrow
question_answer 135) Which one of the following is not a characteristic feature of bryophytes?
A)
Dominant gametophy tic generation
done
clear
B)
Filamentous rhizoids
done
clear
C)
Amphibious habitat
done
clear
D)
Vascular tissues
done
clear
View Answer play_arrow
question_answer 136) Haemophilia is a condition where there is:
A)
no production of haemoglobin in the blood
done
clear
B)
no production of melanin in the skin
done
clear
C)
a failure in the immune mechanism of blood
done
clear
D)
a delay in. the clotting of blood
done
clear
View Answer play_arrow
question_answer 137) Read the statements A and B A: The human small intestine is the longest portion in the alimentary canal B: Absorption of digested food requires a very large surface area Identify the correct choice on the two statements
A)
statement A is correct, B is wrong
done
clear
B)
statement A and B are both correct
done
clear
C)
both the statements are wrong
done
clear
D)
statement B is correct, A is wrong
done
clear
View Answer play_arrow
question_answer 138) In the lac - operon model, lactose molecules function as:
A)
inducers which bind with the operator gene
done
clear
B)
repressers which bind with the operator gene
done
clear
C)
inducers which bind with the represser protein
done
clear
D)
corepressors which bind with represser protein
done
clear
View Answer play_arrow
question_answer 139) When a cell of diameter 2 \[\mu \] grows to double its diameter, what will happen to its surface area volume relationship?
A)
It will reduce to half
done
clear
B)
It will remain the same
done
clear
C)
It cannot be determined
done
clear
D)
It will double
done
clear
View Answer play_arrow
question_answer 140) Which of the following is a genetically dominant trait in human beings?
A)
Colourblindness
done
clear
B)
O blood group
done
clear
C)
Albinism
done
clear
D)
\[R{{h}^{+}}\] blood group
done
clear
View Answer play_arrow
question_answer 141) When the chromosome number of n given organism has one additional chromosome in one of the homologous pairs, the condition is known as:
A)
trisomy
done
clear
B)
monosomy
done
clear
C)
polyploidy
done
clear
D)
nullisomy
done
clear
View Answer play_arrow
question_answer 142) If a germ cell in a female gonad and a germ cell in a male gonad begin undergoing meiosis simultaneously, what will be the ratio of ova and sperms produced?
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
1 : 4
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 143) Soil conservation is a practice in which:
A)
soil is protected from being carried away by wind and water
done
clear
B)
soil is well aerated
done
clear
C)
soil fertility is enhanced
done
clear
D)
soil erosion is allowed
done
clear
View Answer play_arrow
question_answer 144) The main function of lacteals in the villi of human small intestine is the absorption of:
A)
amino acids and glucose
done
clear
B)
glucose and vitamins
done
clear
C)
water and minerals salts
done
clear
D)
fatty acids and glycerol
done
clear
View Answer play_arrow
question_answer 145) A micro-organism, when viewed under a compound microscope with an objective lens of 40 X and an eye piece of 10 X magnification measured 4000 \[\mu \] in length. The same micro-organism when observed under a dissection microscope with a lens of 10 X magnification would measure:
A)
40 \[\mu \]
done
clear
B)
100 \[\mu \]
done
clear
C)
10 \[\mu \]
done
clear
D)
400 \[\mu \]
done
clear
View Answer play_arrow
question_answer 146) Identify from the following, a plant tissue in which lignin does not occur in the cell walls?
A)
Collenchyma
done
clear
B)
Sclerenchyma fibres
done
clear
C)
Sclereids
done
clear
D)
Xylem tracheae
done
clear
View Answer play_arrow
question_answer 147) Which of the following statement is not true with reference to mitochondria?
A)
They divide in synchrony with cell cycle
done
clear
B)
They contain DNA
done
clear
C)
The contain cristae
done
clear
D)
They store and release chemical energy
done
clear
View Answer play_arrow
question_answer 148)
Column - I lists the parts of the human brain and Column - II lists the functions. Match the two columns and identify the correct choice from those given: Column-I Column-II A. Cerebrum p. controls the pituitary B. Cerebellum q. controls vision and hearing C. Hypothalamus r. controls the rate of heart beat D. Midbrain s. seat of intelligence t. maintains body posture
A)
A = t; B = s; C = q; D = p
done
clear
B)
A = s; B = t; C = q; D = p
done
clear
C)
A = t; B = s; C = p; D = q
done
clear
D)
A = s; B = t; C = p; D = q
done
clear
View Answer play_arrow
question_answer 149) The site of EMP pathway of breakdown of glucose in a cell is
A)
nucleoplasm
done
clear
B)
mitochondria
done
clear
C)
cytoplasm
done
clear
D)
peroxisome
done
clear
View Answer play_arrow
question_answer 150)
The following diagram indicates the reflex arc. Identify the parts labelled as A, B, C, D, E, F and G. Choose the correct option:
A)
A = sense organ; B = sensory nerve
C = dorsal horn; D = interneuron
E = ventral horn; F = motor nerve
G = effector
done
clear
B)
A = sense organ; B = sensory nerve
C = ventral horn; D = interneuron
E = dorsal horn; F = motor nerve
G = effector
done
clear
C)
A = sense organ; B = motor nerve
C = dorsal horn; D = interneuron
E = ventral horn; F = sensory nerve
G = effector
done
clear
D)
A = effector; B = motor nerve
C = ventral horn; D = interneuron;
E = dorsal horn; F = sensory nerve;
G = sense organ
done
clear
View Answer play_arrow
question_answer 151) Which of the following tissue originates exclusively from the ectoderm of the embryo?
A)
Muscular tissue
done
clear
B)
Epithelial tissue
done
clear
C)
Nervous tissue
done
clear
D)
Connective tissue
done
clear
View Answer play_arrow
question_answer 152) The pyramid of energy is always upright for any ecosystem. This situation indicates the fact that:
A)
producers have the lowest energy conversion efficiency
done
clear
B)
carnivores have a better energy conversion efficiency than herbivores
done
clear
C)
energy conversion efficiency is the same in all trophic levels
done
clear
D)
herbivores have a better energy conversion efficiency than carnivores
done
clear
View Answer play_arrow
question_answer 153) Gynoecium in the members of family Leguminosae is composed of:
A)
two carpels
done
clear
B)
one carpel
done
clear
C)
five carpels
done
clear
D)
three carpels
done
clear
View Answer play_arrow
question_answer 154) Identify from the following, the compound that links glycolysis and Krebs cycle:
A)
oxalo acetic acid
done
clear
B)
pvruvic acid
done
clear
C)
lactic acid
done
clear
D)
acetyl Co-A
done
clear
View Answer play_arrow
question_answer 155) Which part of the human brain controls the breathing movements?
A)
Medulla oblongata
done
clear
B)
Cerebellum
done
clear
C)
Diencephalon
done
clear
D)
Cerebrum
done
clear
View Answer play_arrow
question_answer 156) Leaf fall occurs in a tree when there is an increase in the concentration of:
A)
abscisic acid
done
clear
B)
auxins
done
clear
C)
gibberellins
done
clear
D)
cytokinins
done
clear
View Answer play_arrow
question_answer 157) Which of the following is a disease resistant, high yielding breed of poultry developed in Karnataka?
A)
Aseel
done
clear
B)
White leg horn
done
clear
C)
Giriraja
done
clear
D)
Plymouth rock
done
clear
View Answer play_arrow
question_answer 158) Sertoli cells are nourishing cells in the testis. They also secrete a hormone. Identify the same:
A)
gonadotropin
done
clear
B)
testosterone
done
clear
C)
relaxin
done
clear
D)
inhibin
done
clear
View Answer play_arrow
question_answer 159) Molecular biology is concerned with the study of:
A)
structure and functions of polymers of life
done
clear
B)
all aspects of micro organisms
done
clear
C)
the process by which molecules of chemical substances organized into primitive form of life
done
clear
D)
the chemistry of living organisms
done
clear
View Answer play_arrow
question_answer 160) The inner, darker and harder portion of secondary xylem that cannot conduct water, in an older dicot stem, is called:
A)
alburnum
done
clear
B)
bast
done
clear
C)
wood
done
clear
D)
duramen
done
clear
View Answer play_arrow
question_answer 161) The closed circulatory system is found in:
A)
insects
done
clear
B)
lobsters
done
clear
C)
frog
done
clear
D)
clams
done
clear
View Answer play_arrow
question_answer 162) The law of limiting factors was proposed with particular reference to photosynthesis. Identify the scientist who proposed this law?
A)
Calvin
done
clear
B)
Weismann
done
clear
C)
Emerson
done
clear
D)
Blackman
done
clear
View Answer play_arrow
question_answer 163) Osmo regulation in Paramecium is a function of:
A)
contractile vacuole
done
clear
B)
trichocysts
done
clear
C)
cytopyge
done
clear
D)
cytostome
done
clear
View Answer play_arrow
question_answer 164) Identify from the following the branch of biology which provides direct evidences in favour of organic evolution?
A)
Morphology
done
clear
B)
Taxonomy
done
clear
C)
Palaeontology
done
clear
D)
Embryology
done
clear
View Answer play_arrow
question_answer 165) Which of the following groups of cells in the male gonad, represent haploid cells?
A)
Spermatogonial cells
done
clear
B)
Germinal epithelia cells
done
clear
C)
Secondary spermatocytes
done
clear
D)
Primary spermatocytes
done
clear
View Answer play_arrow
question_answer 166) A nucleosome is a pertion of the chronionema containing:
A)
only histones
done
clear
B)
both DNA and histones
done
clear
C)
only DNA
done
clear
D)
both DNA and RNA
done
clear
View Answer play_arrow
question_answer 167) Maximum amount of oxygen is exchanged from the blood in the:
A)
capillaries surrounding tissue cells
done
clear
B)
arteries of the body
done
clear
C)
left auricle of the heart
done
clear
D)
capillaries surrounding the alveoli
done
clear
View Answer play_arrow
question_answer 168) Which of the following term is used to describe the component isolated from a plant, for in vitro culturing in the specific medium?
A)
Callus
done
clear
B)
Embryoid
done
clear
C)
Synthetic seeds
done
clear
D)
Explant
done
clear
View Answer play_arrow
question_answer 169) If a cell has twice as much DNA as in a normal functional cell, it means that the cells:
A)
is preparing to divide
done
clear
B)
has completed division
done
clear
C)
has reached the end of its life span
done
clear
D)
has ceased to function
done
clear
View Answer play_arrow
question_answer 170) Apical dominance in plants is due to the presence of:
A)
cytokinins in the leaf apex
done
clear
B)
gibberellins in the lateral bud
done
clear
C)
auxins at the shoot tip
done
clear
D)
abscisic acid at the shoot tip
done
clear
View Answer play_arrow
question_answer 171) Which of the following structures are derivatives of the endoderm?
A)
Alimentary canal and respiratory structures
done
clear
B)
Muscles and blood
done
clear
C)
Excretory and reproductive structures
done
clear
D)
Skin and nerve cord
done
clear
View Answer play_arrow
question_answer 172) The sequence of nitrogen bases in a portion of a coding segment of DNA was AAT GOT TAG GCA. What will be the sequence of nitrogen bases in the corresponding region of the transcribed m -RNA?
A)
UUT CGT TUC CGU
done
clear
B)
AAT GCT TAG GCA
done
clear
C)
UUA CGA AUC CGU
done
clear
D)
TTA CGA ATC CGT
done
clear
View Answer play_arrow
question_answer 173) Which chamber of the human heart has the thickest muscular wall?
A)
Left auricle
done
clear
B)
Left ventricle
done
clear
C)
Right auricle
done
clear
D)
Right ventricle
done
clear
View Answer play_arrow
question_answer 174) Entomology is concerned with the study of:
A)
formation and properties of soil
done
clear
B)
agricultural practices
done
clear
C)
various aspects of human life
done
clear
D)
various aspects of insects
done
clear
View Answer play_arrow
question_answer 175) Which of the following is called as a detritivore?
A)
An animal feeding on decaying organic matter
done
clear
B)
An animal feeding on a plant
done
clear
C)
A plant feeding on an animal
done
clear
D)
An animal feeding on another animal
done
clear
View Answer play_arrow
question_answer 176) How many human teeth appear twice during the life span of an individual?
A)
16
done
clear
B)
32
done
clear
C)
22
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 177) If the size of a fertilized egg of frog is compared with the size of its blastula and gastrula stages, which of the following observations will be correct?
A)
There is a progressive increase in size from zygote to blastula to gastrula
done
clear
B)
All the three will be of the same size
done
clear
C)
Zygote will be smaller, while blastula and gastrula will be larger
done
clear
D)
Gastrula will be larger, while zygote and blastula will be of same size
done
clear
View Answer play_arrow
question_answer 178) During protein synthesis AUG functions as the initiator codon in; m - RNA. What should be the anticodon on the? t - RNA molecule that picks up and brings the amino acid specified by the codon?
A)
UAC
done
clear
B)
TAC
done
clear
C)
CAU
done
clear
D)
GUA
done
clear
View Answer play_arrow
question_answer 179) Choose the odd pair out in the following:
A)
areolar connective tissue - collagen
done
clear
B)
epithelium - keratin
done
clear
C)
neuron - melanin
done
clear
D)
muscle fibre - actin
done
clear
View Answer play_arrow
question_answer 180) The macronutrient which is an essential component of all organic compounds, yet not obtained by plants from soil, is:
A)
nitrogen
done
clear
B)
carbon
done
clear
C)
phosphorus
done
clear
D)
magnesium
done
clear
View Answer play_arrow