question_answer 1) SI unit of magnetic flux is:
A)
tesla
done
clear
B)
oersted
done
clear
C)
weber
done
clear
D)
Gauss
done
clear
View Answer play_arrow
question_answer 2) A body of mass m is moving towards east and another body of equal mass is moving towards north. If after collision both stick together, their speed after collision would be:
A)
\[v\]
done
clear
B)
\[v/2\]
done
clear
C)
\[\sqrt{2}v\]
done
clear
D)
\[v/2\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 3) A body of mass 1 kg is moving in a vertical circular path of radius 1 m. The difference between the kinetic energies at its highest and lowest position is :
A)
20 J
done
clear
B)
10 J
done
clear
C)
\[4\sqrt{5}J\]
done
clear
D)
10\[(\sqrt{5}-1)J\]
done
clear
View Answer play_arrow
question_answer 4) Across each of two capacitors of capacitance \[1\mu F,\]a potential difference of 10 V is applied. Then positive plate of one is connected to the negative plate of the other, and negative plate of one is connected to the positive plate of the other. After contact:
A)
charge on each is zero
done
clear
B)
charge on each is same but non-zero
done
clear
C)
charge on each is different but non-zero
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 5) Magnification of a compound microscope is 30. Focal length of eyepiece is 5 cm and the image is formed at a distance of distinct vision 25 cm. The magnification of the objective lens is :
A)
6
done
clear
B)
5
done
clear
C)
7.5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 6) Kirchhoffs law of junction, \[\sum I=0,\] is based on:
A)
conservation of energy
done
clear
B)
conservation of charge
done
clear
C)
conservation of energy as well as charge
done
clear
D)
conservation of momentum
done
clear
View Answer play_arrow
question_answer 7) Calculate the amount of heat (in calories) required to convert 5 g of ice at \[{{0}^{o}}C\] to steam at \[{{100}^{o}}C\].
A)
3100
done
clear
B)
3200
done
clear
C)
3600
done
clear
D)
4200
done
clear
View Answer play_arrow
question_answer 8) A transverse wave is expressed as, \[y={{y}_{0}}\sin \,2\pi \,ft\]. For what value of \[\lambda .\] maximum particle velocity equals to 4 times the wave velocity?
A)
\[{{y}_{0}}\pi /2\]
done
clear
B)
\[2{{y}_{0}}\pi \]
done
clear
C)
\[{{y}_{0}}\pi \]
done
clear
D)
\[{{y}_{0}}\pi /4\]
done
clear
View Answer play_arrow
question_answer 9) Two bodies are thrown up at angles of \[{{40}^{o}}\] and \[{{60}^{o}}\] respectively, with the horizontal, if both bodies attain same vertical height, then ratio of velocities with which these are thrown is :
A)
\[\sqrt{2/3}\]
done
clear
B)
\[2/\sqrt{3}\]
done
clear
C)
\[\sqrt{3}/2\]
done
clear
D)
\[\sqrt{3}/2\]
done
clear
View Answer play_arrow
question_answer 10) Charges 4 Q, q and Q are placed along x-axis at position \[x=o,x=l/2\] and\[x=l,\] respectively. Find the value of q, so that force on charge Q is zero.
A)
\[Q\]
done
clear
B)
\[\frac{Q}{2}\]
done
clear
C)
\[-\frac{Q}{2}\]
done
clear
D)
\[-Q\]
done
clear
View Answer play_arrow
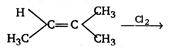
question_answer 11)
A ray falls on a prism ABC.(AB = BC) and travels as shown in figure. The least value of refractive index of material of the prism, should be:
A)
1.5
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
1.33
done
clear
D)
\[\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 12) Escape velocity from a planet is \[{{V}_{e}}\]. If its mass is increased to 8 times and its radius is increased to 2 times, then the new escape velocity would be:
A)
\[{{V}_{e}}\]
done
clear
B)
\[\sqrt{2}{{v}_{e}}\]
done
clear
C)
\[2{{v}_{e}}\]
done
clear
D)
\[2\sqrt{2}{{v}_{e}}\]
done
clear
View Answer play_arrow
question_answer 13) A body takes time t to reach the bottom of an inclined plane of angle \[\theta \] with the horizontal. If the plane is made rough, time taken now is it. The coefficient of friction of the rough surface is:
A)
\[\frac{3}{4}\tan \theta \]
done
clear
B)
\[\frac{2}{3}\tan \theta \]
done
clear
C)
\[\frac{1}{4}\tan \theta \]
done
clear
D)
\[\frac{1}{2}\tan \theta \]
done
clear
View Answer play_arrow
question_answer 14) Two small charged spheres A and B have charges \[10\,\mu \,C\] and \[40\,\mu \,C\], respectively and are held at a separation of 90 cm from each other At what distance from A, electric intensity would be zero?
A)
22.5cm
done
clear
B)
18cm
done
clear
C)
36 cm
done
clear
D)
30 cm
done
clear
View Answer play_arrow
question_answer 15) 50 tuning forks are arranged in increasing order of their frequencies such that each gives 4 beats/s with its previous tuning fork. If the frequency of the last fork is octave of the First, then the frequency of the first tuning fork is :
A)
200 Hz
done
clear
B)
204 Hz
done
clear
C)
196 Hz
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 16) In a cyclotron, if a deuteron can gain an energy of 40MeV, then a proton can gain an energy of:
A)
40MeV
done
clear
B)
80MeV
done
clear
C)
20MeV
done
clear
D)
60MeV
done
clear
View Answer play_arrow
question_answer 17) Graph between velocity and displacement of a panicle, executing SHM is :
A)
a straight line
done
clear
B)
a parabola
done
clear
C)
a hyperbola
done
clear
D)
an ellipse
done
clear
View Answer play_arrow
question_answer 18) In the nuclear reaction, \[_{72}{{X}^{180}}\xrightarrow{-\alpha }Y\xrightarrow{-\beta }Z\xrightarrow{-\alpha }A\xrightarrow{-\gamma }p\]the atomic mass and atomic number of P are, respectively :
A)
170, 69
done
clear
B)
172, 69
done
clear
C)
172,70
done
clear
D)
170,70
done
clear
View Answer play_arrow
question_answer 19) A radioactive substance has activity 64 times higher than the required normal level. If \[{{T}_{1}}_{/2}=2h,\] then the time after which it should be possible to work with it, is :
A)
16 h
done
clear
B)
6h
done
clear
C)
10 h
done
clear
D)
12 h
done
clear
View Answer play_arrow
question_answer 20) An electron, moving in a uniform magnetic field of induction of intensity B, has its radius directly proportional to :
A)
its charge
done
clear
B)
magnetic field
done
clear
C)
speed
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 21) The apparent frequency in Dopplers effect does not depend upon :
A)
speed of the observer
done
clear
B)
distance between observer and source
done
clear
C)
speed of the source
done
clear
D)
frequency from the source
done
clear
View Answer play_arrow
question_answer 22) Two simple pendulums whose lengths are 100 cm and 121 cm are suspended side by side. Their bobs are pulled together and the released. After how many minimum oscillations of the longer pendulum, will the two be in phase again?
A)
11
done
clear
B)
10
done
clear
C)
21
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 23) If percentage change in current through resistor is 1%, then the change in power through it would be :
A)
1%
done
clear
B)
2%
done
clear
C)
1.7%
done
clear
D)
0.5%
done
clear
View Answer play_arrow
question_answer 24) Three identical bulbs are connected in series and these together dissipate a power P. If now the bulbs are connected in parallel, then a power dissipated will be :
A)
P/3
done
clear
B)
3P
done
clear
C)
9P
done
clear
D)
P/9
done
clear
View Answer play_arrow
question_answer 25) Acceleration due to gravity:
A)
decreases from equator to poles
done
clear
B)
decreases from poles to equator
done
clear
C)
is maximum at the centre of the earth
done
clear
D)
is maximum at the equator
done
clear
View Answer play_arrow
question_answer 26) Two bodies having masses \[{{m}_{1}}=40\,g\] and \[{{m}_{2}}=60\,g\] are attached to the ends of a string of negligible mass and suspended from massless pulley. The acceleration of the bodies is:
A)
\[1\,m/{{s}^{2}}\]
done
clear
B)
\[2\,\,m/{{s}^{2}}\]
done
clear
C)
\[0.4\,\,m/{{s}^{2}}\]
done
clear
D)
\[4\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) A block is moving up an inclined plane of inclination \[{{60}^{o}}\] with velocity of 20 m/s and stops after 2s. If \[g=10\,m/{{s}^{2}}\], then the approximate value of coefficient of friction is
A)
3
done
clear
B)
3.3
done
clear
C)
0.27
done
clear
D)
0.33
done
clear
View Answer play_arrow
question_answer 28) A coin placed on a rotating turntable just slips, if it is placed at a distance of 8 cm from the centre. If angular speed of the turntable is doubled, it will just slip at a distance of:
A)
1 cm
done
clear
B)
2 cm
done
clear
C)
4 cm
done
clear
D)
8 cm
done
clear
View Answer play_arrow
question_answer 29) When a man increases his speed by 2 m/s, he finds that his kinetic energy is doubled, the original speed of the man is :
A)
\[(2\sqrt{2}-1)m/s\]
done
clear
B)
\[(2\sqrt{2}+1)m/s\]
done
clear
C)
\[4.5\,m/s\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 30) A ball falling freely from a height of 4.9 m/s hits a horizontal surface. If \[e\frac{3}{4},\] then the ball will hit the surface second time after :
A)
0.5 s
done
clear
B)
1.5 s
done
clear
C)
3.5 s
done
clear
D)
3.4 s
done
clear
View Answer play_arrow
question_answer 31) The distance between the sun and the earth be r, then the angular momentum of the earth around the sun is proportional to :
A)
\[\sqrt{r}\]
done
clear
B)
\[{{r}^{3/2}}\]
done
clear
C)
\[r\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 32) A body executing SHM has its velocity 10 cm/s and 7 cm/s, when its displacements from the mean position are 3 cm and 4 cm, respectively The length of path is :
A)
10cm
done
clear
B)
9.5cm
done
clear
C)
4cm
done
clear
D)
11.36cm
done
clear
View Answer play_arrow
question_answer 33) A 700 g solid cube having an edge of length 10 cm floats in water. The volume of cube outside water is:
A)
\[2.4\,c{{m}^{3}}\]
done
clear
B)
\[4.8\,c{{m}^{3}}\]
done
clear
C)
\[300\,c{{m}^{3}}\]
done
clear
D)
\[500\,c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 34) A 1m long steel wire of cross-sectional area \[1\,m{{m}^{2}}\] is extended by 1 mm. If\[Y=2\times {{10}^{11}}N/{{m}^{2}}\], then the work done is :
A)
0.1 J
done
clear
B)
0.2 J
done
clear
C)
0.3 J
done
clear
D)
0.4 J
done
clear
View Answer play_arrow
question_answer 35) The slit width, when a light of wavelength \[6500\overset{\text{o}}{\mathop{\text{A}}}\,\] is incident on a slit, if first minima for red light is at \[{{30}^{o}}\], is:
A)
\[1\times {{10}^{-6}}\,m\]
done
clear
B)
\[5.2\times {{10}^{-6}}\,m\]
done
clear
C)
\[1.3\times {{10}^{-6}}\,m\]
done
clear
D)
\[2.6\times {{10}^{-6}}\,m\]
done
clear
View Answer play_arrow
question_answer 36) A light source approaches the observer with velocity 0.5c. Dopplers shift for light of wavelength \[5500\,\overset{o}{\mathop{A}}\,\] is :
A)
\[616\,\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[616\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[5500\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[6160\,\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 37) A charged particle moves in an electric field from A to B then from B to A :
A)
If \[{{W}_{AB}}>{{W}_{BA,}}\] then the field is conservative
done
clear
B)
If \[{{W}_{AB}}+{{W}_{BA}}=0\], then the field is conservative
done
clear
C)
If \[{{W}_{AB}}+{{W}_{BA}}>0\], then the field is conservative
done
clear
D)
If \[{{W}_{AB}}={{W}_{BA}}\], then the field is conservative
done
clear
View Answer play_arrow
question_answer 38) A solid metal sphere of radius 50 cm carries a charge\[25\times {{10}^{-10}}C\]. The electrostatic potential at a distance of 20 cm from the centre will be:
A)
25V
done
clear
B)
15V
done
clear
C)
35V
done
clear
D)
45V
done
clear
View Answer play_arrow
question_answer 39) Two plates (area = S) charged to \[+{{q}_{1}}\] and \[+{{q}_{2}}({{q}_{2}}<{{q}_{1}})\] brought closer to form a capacitor of capacitance C. The potential difference across the plates is:
A)
\[\frac{{{q}_{1}}-{{q}_{2}}}{2C}\]
done
clear
B)
\[\frac{{{q}_{1}}-{{q}_{2}}}{C}\]
done
clear
C)
\[\frac{{{q}_{1}}-{{q}_{2}}}{4C}\]
done
clear
D)
\[\frac{2({{q}_{1}}-{{q}_{2}})}{C}\]
done
clear
View Answer play_arrow
question_answer 40) What is the drift velocity of electrons, if the current flowing through a copper wire of 1 mm diameter is 1.1A? Assume that each atom of copper contributes one electron. (Given, density of \[Cu=9\,\,g/c{{m}^{3}}\] and atomic weight of \[Cu=63\])
A)
0.3mm/s
done
clear
B)
0.Smm/s
done
clear
C)
0.lmm/s
done
clear
D)
0.2mm/s
done
clear
View Answer play_arrow
question_answer 41) A uniform magnetic field is at right angle to the direction of motion of proton. As a result, the proton describes a circular path of radius 2.5 cm. If the speed of proton is doubled, then the radius of the circular path will be :
A)
0.5cm
done
clear
B)
2.5cm
done
clear
C)
5.0cm
done
clear
D)
7.5cm
done
clear
View Answer play_arrow
question_answer 42) An electron accelerated through a potential difference enters into a uniform transverse magnetic field and experiences a force F. If the accelerating potential is increased to 2 V, the electron in the same magnetic field will experience a force :
A)
\[F\]
done
clear
B)
\[F/2\]
done
clear
C)
\[\sqrt{2}F\]
done
clear
D)
\[2F\]
done
clear
View Answer play_arrow
question_answer 43) The certain amount of current when flowing in a properly set tangent galvanometer, produces a deflection of \[{{45}^{o}}\]. The current be reduced by a factor of \[\sqrt{3}\], the deflection would :
A)
decrease by \[{{30}^{o}}\]
done
clear
B)
decrease by \[{{15}^{o}}\]
done
clear
C)
increase by \[{{15}^{o}}\]
done
clear
D)
increase by \[{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 44) If a current of 3 A flowing in the primary coil is reduced to zero in 0.01 s, then the induced emf in the secondary coil is 1500 V. The mutual inductance between the two coils is :
A)
0.5 H
done
clear
B)
5H
done
clear
C)
1.5H
done
clear
D)
10 H
done
clear
View Answer play_arrow
question_answer 45) In order to obtain time constant of 10 s in an R - C circuit containing a resistance of \[{{10}^{3}}\,\Omega \]the capacity of the condenser should be :
A)
\[10\mu F\]
done
clear
B)
\[100\mu F\]
done
clear
C)
\[1000\mu F\]
done
clear
D)
\[10000\mu F\]
done
clear
View Answer play_arrow
question_answer 46) An a-particle when accelerated through a potential difference of V volt has a wavelength \[\lambda \] associated with it. In order to have same \[\lambda \] by what potential difference a proton must be accelerated?
A)
8V
done
clear
B)
6V
done
clear
C)
4V
done
clear
D)
12V
done
clear
View Answer play_arrow
question_answer 47) Hailstone at \[{{0}^{o}}C\] falls from a height of 1 km on an insulating surface converting whole of its kinetic energy into heat. What part of it will melt? \[(g=10\,m/{{s}^{2}})\] (Latent heat of ice \[=3.34\times {{10}^{5}}J/kg\])
A)
\[\frac{1}{33}\]
done
clear
B)
\[\frac{1}{18}\]
done
clear
C)
\[\frac{3}{3}\times {{10}^{-4}}\]
done
clear
D)
All of it will melt
done
clear
View Answer play_arrow
question_answer 48) At \[{{27}^{o}}C\], a motor car tyre has pressure of 2 atm. The temperature at which the tyre suddenly burst will be : (Given, \[{{\gamma }_{air}}=1.4\])
A)
246.1K
done
clear
B)
250 K
done
clear
C)
290K
done
clear
D)
248 K
done
clear
View Answer play_arrow
question_answer 49) A flat mirror revolves at a constant angular velocity making 2rev/s. With what velocity will a light spot move along a spherical screen with a radius of 10 m, if the mirror is at a centre of curvature of the screen?
A)
251.2 m/s
done
clear
B)
261.2m/s
done
clear
C)
.271.2m/s
done
clear
D)
241.2m/s
done
clear
View Answer play_arrow
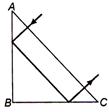
question_answer 50)
A vessel consists of two plane mirrors at right angle (as shown in figure). The vessel is filled with water. The total deviation in incident ray
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{180}^{o}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 51) Which of the following is not an actinide?
A)
Curium
done
clear
B)
Californium
done
clear
C)
Uranium
done
clear
D)
Terbium
done
clear
View Answer play_arrow
question_answer 52) Europium is :
A)
s-block element
done
clear
B)
p-block element
done
clear
C)
d-block element
done
clear
D)
\[f\]-block element
done
clear
View Answer play_arrow
question_answer 53) For an electron, if the uncertainty in velocity is Av, the uncertainty in its position \[\Delta v\], the uncertainty in its position \[(\Delta x)\] is given by :
A)
\[\frac{hm}{4\pi \Delta v}\]
done
clear
B)
\[\frac{4\pi }{hm\Delta v}\]
done
clear
C)
\[\frac{h}{4\pi m\Delta v}\]
done
clear
D)
\[\frac{h\pi m}{h\Delta v}\]
done
clear
View Answer play_arrow
question_answer 54) The reagent in Friedel-Crafts reaction is :
A)
pyridine
done
clear
B)
\[RCOCl\]
done
clear
C)
\[RCOOH\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 55) The \[{{K}_{sp}}\] of \[Mg{{(OH)}_{2}}\] is \[1\times {{10}^{-12}}.0.001\,Mg\]\[{{(OH)}_{2}}\] will precipitate at the limited pH :
A)
3
done
clear
B)
9
done
clear
C)
5
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 56) Equation of Boyles law is :
A)
\[\frac{dP}{P}=-\frac{dV}{V}\]
done
clear
B)
\[\frac{dP}{P}=+\frac{dV}{V}\]
done
clear
C)
\[\frac{{{d}^{2}}P}{P}=-\frac{dV}{dT}\]
done
clear
D)
\[\frac{{{d}^{2}}P}{P}=+\frac{{{d}^{2}}V}{dT}\]
done
clear
View Answer play_arrow
question_answer 57) A radioactive sample is emitting 64 times radiations than non-hazardous limit. If its half life is 2h, after what time it becomes non-hazardous?
A)
16 h
done
clear
B)
12 h
done
clear
C)
8h
done
clear
D)
4h
done
clear
View Answer play_arrow
question_answer 58) A metal surface is exposed to solar radiations:
A)
the emitted electrons have energy less than a maximum value of energy depending upon frequency of incident radiations
done
clear
B)
the emitted electrons have energy less than maximum value of energy depending . upon intensity of incident radiations
done
clear
C)
the emitted electrons have zero energy
done
clear
D)
the emitted electrons have energy equal to energy of photons of incident light
done
clear
View Answer play_arrow
question_answer 59) Which of the following transitions have minimum wavelengths?
A)
\[{{n}_{4}}\to {{n}_{1}}\]
done
clear
B)
\[{{n}_{2}}\to {{n}_{1}}\]
done
clear
C)
\[{{n}_{4}}\to {{n}_{2}}\]
done
clear
D)
\[{{n}_{3}}\to {{n}_{1}}\]
done
clear
View Answer play_arrow
question_answer 60) Orbital is :
A)
circular path around the nucleus in which the electrons revolves
done
clear
B)
space around the nucleus where the probablity of finding the electron is maximum
done
clear
C)
amplitude of electron wave
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 61) Number of unpaired electrons in \[M{{n}^{4+}}\] is :
A)
3
done
clear
B)
5
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 62) Which of the following sequence is correct as per aufbau principle?
A)
\[3s<3d<4s<4p\]
done
clear
B)
\[1s<2p<4s<3d\]
done
clear
C)
\[2s<5s<4p<5d\]
done
clear
D)
\[2s<2p<3d<3p\]
done
clear
View Answer play_arrow
question_answer 63) Ionic compounds are formed most easily with:
A)
low electron affinity, high ionisation energy
done
clear
B)
high electron affinity, low ionisation energy
done
clear
C)
low electron affinity, low ionisation energy
done
clear
D)
high electron affinity, high ionisation energy
done
clear
View Answer play_arrow
question_answer 64) The enthalpy change \[(\Delta H)\] for the neutralisation of \[M\,\,HCl\] by caustic potash in dilute solution at 298 K is :
A)
68 kJ
done
clear
B)
65 kJ
done
clear
C)
57.3 kJ
done
clear
D)
50 kJ
done
clear
View Answer play_arrow
question_answer 65) Which of the following is not hydrolysed?
A)
\[AsC{{l}_{3}}\]
done
clear
B)
\[P{{F}_{3}}\]
done
clear
C)
\[SbC{{l}_{3}}\]
done
clear
D)
\[N{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following gas is linear?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 67) \[N{{H}_{4}}COON{{H}_{2}}(s)2N{{H}_{3}}(g)+C{{O}_{2}}(g)\] if equilibrium pressure is 3 aim for the above reaction. \[{{K}_{p}}\] for the reaction is:
A)
4
done
clear
B)
27
done
clear
C)
4/27
done
clear
D)
1/27
done
clear
View Answer play_arrow
question_answer 68) Number of isomeric primary amines obtained from \[{{C}_{4}}{{H}_{11}}N\] are :
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 69) If hydrogen electrode dipped in 2 solution of \[pH=3\] and\[pH=6\] and salt bridge is connected the emf of resulting cell is:
A)
0,177V
done
clear
B)
- 0.3V
done
clear
C)
0.052V
done
clear
D)
0.104V
done
clear
View Answer play_arrow
question_answer 70) A radioactive nucleus will not emit:
A)
alpha and beta rays simultaneously
done
clear
B)
beta and gamma rays simultaneously
done
clear
C)
gamma and alpha rays
done
clear
D)
gamma rays only
done
clear
View Answer play_arrow
question_answer 71) In face centred cubic unit cell edge length is:
A)
\[\frac{4}{\sqrt{3}}r\]
done
clear
B)
\[\frac{4}{\sqrt{2}}r\]
done
clear
C)
\[2\,r\]
done
clear
D)
\[\frac{\sqrt{3}}{2}\,r\]
done
clear
View Answer play_arrow
question_answer 72) If the \[Z{{n}^{2+}}/Zn\] electrode is diluted to 100 times than the change in emf:
A)
increase of 59 mV
done
clear
B)
decrease of 59 mV
done
clear
C)
increase of 29.5 mV
done
clear
D)
decrease of 29.5 mV
done
clear
View Answer play_arrow
question_answer 73) Which of the following reactions end infinite time?
A)
Zero order
done
clear
B)
1st order
done
clear
C)
2nd order
done
clear
D)
3rd order
done
clear
View Answer play_arrow
question_answer 74) If equivalent conductance of 1M benzoic acid is \[12.8\,oh{{m}^{-1}}c{{m}^{2}}\] and if the conductance of benzoate ion and \[{{H}^{+}}\] ion are 42 and \[288.42\,oh{{m}^{-1}}c{{m}^{2}}\] respectively. Its degree of dissociation is :
A)
39%
done
clear
B)
3.9%
done
clear
C)
0.35%
done
clear
D)
0.039%
done
clear
View Answer play_arrow
question_answer 75) In which of the following reactions carbon-carbon bond formation takes place?
A)
Cannizaro reaction
done
clear
B)
Reimer-Tiemann reaction
done
clear
C)
HVZ reaction
done
clear
D)
Schmidt reaction
done
clear
View Answer play_arrow
question_answer 76) Which gives only mono-substituted product?
A)
o-dinitrobenzene
done
clear
B)
m-dinitrobenzene
done
clear
C)
p-dinitrobenzene
done
clear
D)
Nitrobenzene
done
clear
View Answer play_arrow
question_answer 77) Which of the following is most polarized?
A)
Kr
done
clear
B)
He
done
clear
C)
Ar
done
clear
D)
Xe
done
clear
View Answer play_arrow
question_answer 78) Order of boiling point is :
A)
\[HF>HI>HBr>HCl\]
done
clear
B)
\[HF>HBr>HI>HCl\]
done
clear
C)
\[HCl>HBr>HI>HF\]
done
clear
D)
\[HCl>HI>HBr>HF\]
done
clear
View Answer play_arrow
question_answer 79) If two substance A and B have \[{{P}_{{{A}^{o}}}}:{{P}_{{{B}^{o}}}}=1:2\]and have mole fraction in solution 1 : 2 then mol fraction of A in vapours :
A)
0.33
done
clear
B)
0.25
done
clear
C)
0.52
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 80) Order of hydrolysis for the following : (I) \[RCOCl\] (II) \[RCOOR\] (III) \[RCON{{H}_{2}}\] (IV) \[{{(RCO)}_{2}}O\]
A)
\[1>IV>II>III\]
done
clear
B)
\[1>II>III>IV\]
done
clear
C)
\[1>III>II>IV\]
done
clear
D)
\[1V>III>II>I\]
done
clear
View Answer play_arrow
question_answer 81) If an aqueous solution of glucose is allowed to freeze than crystal of which will be separated out first?
A)
glucose
done
clear
B)
Water
done
clear
C)
Both [a] & [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) \[CH\equiv CH\xrightarrow[{{H}_{2}}S{{O}_{4}}]{HgS{{O}_{4}}}A\xrightarrow[{{H}_{2}}O]{C{{H}_{3}}MgBr}B\]\[\xrightarrow{P/B{{r}_{2}}}C\]Here C is:
A)
\[C{{H}_{3}}CH(Br)C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\]
done
clear
C)
\[C{{H}_{2}}=CH-Br\]
done
clear
D)
\[BrCH=CH-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 83) Number of bonds in benzene :
A)
\[6\,\sigma \] and \[3\,\pi \]
done
clear
B)
\[12\,\sigma \] and \[3\,\pi \]
done
clear
C)
\[3\,\sigma \] and \[12\,\pi \]
done
clear
D)
\[6\,\sigma \] and \[6\,\pi \]
done
clear
View Answer play_arrow
question_answer 84) If the \[{{V}_{rms}}\] is \[30\,{{R}^{1/2}}\] at \[{{27}^{o}}C\] then calculate the molar mass of gas in kilogram :
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
0.001
done
clear
View Answer play_arrow
question_answer 85) If the enolate ion combines with carbonyl group of ester, we get:
A)
aldol
done
clear
B)
\[\alpha ,\beta \]-unsaturated ester
done
clear
C)
\[\beta \]-keto ester
done
clear
D)
acid
done
clear
View Answer play_arrow
question_answer 86)
A)
enantiomers
done
clear
B)
diastereomers
done
clear
C)
meso compound
done
clear
D)
identical
done
clear
View Answer play_arrow
question_answer 87) The standard molar heat of formation of ethane. \[C{{O}_{2}}\] and water are respectively -21.1, -94.1 and-68.3 kcal. The standard molar heat of combustion of ethane will be:
A)
- 372 kcal
done
clear
B)
162 kcal
done
clear
C)
-240 kcal
done
clear
D)
183.5 kcal
done
clear
View Answer play_arrow
question_answer 88) \[_{72}{{X}^{180}}\xrightarrow{2\alpha }\xrightarrow{\beta }{{\xrightarrow{\gamma }}_{z}}{{X}^{A}},Z,A\] are:
A)
69, 172
done
clear
B)
172, 69
done
clear
C)
180, 70
done
clear
D)
182, 68
done
clear
View Answer play_arrow
question_answer 89) \[A+2B\xrightarrow{{}}C+D\], if \[-\xrightarrow[dt]{d[A]}=5\times {{10}^{-4}}\] \[mol\,{{L}^{-1}}{{s}^{-1}}\], then \[-\frac{d[B]}{dt}\] is:
A)
\[2.5\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[5.0\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[2.5\times {{10}^{-3}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[1.0\times {{10}^{-3}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following is paramagnetic?
A)
\[{{N}_{2}}\]
done
clear
B)
\[{{C}_{2}}\]
done
clear
C)
\[{{C}_{2}}^{+}\]
done
clear
D)
\[{{O}_{2}}^{2-}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following compounds will react with \[NaHC{{O}_{3}}\] solution to give sodium salt and carbon dioxide?
A)
Acetic acid
done
clear
B)
n-hexanol
done
clear
C)
Phenol
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 92) Which will give chiral molecule?
A)
\[C{{H}_{3}}COCl\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}CHO\xrightarrow[{{H}^{+}}/{{H}_{2}}O]{C{{H}_{3}}MgBr}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CH{{C}_{2}}{{H}_{5}}\xrightarrow{Cu}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) Which is not a polymer?
A)
Sucrose
done
clear
B)
Enzyme
done
clear
C)
Starch
done
clear
D)
Teflon
done
clear
View Answer play_arrow
question_answer 94) Which statement is wrong for NO?
A)
It is anhydride of nitrous acid
done
clear
B)
Its dipole moment is 0.22 D
done
clear
C)
It forms dimer
done
clear
D)
It is paramagnetic
done
clear
View Answer play_arrow
question_answer 95) Which of the following reactions will not give propane?
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl\xrightarrow[{{H}_{2}}O]{Mg/ether}\]
done
clear
B)
\[C{{H}_{3}}COCl\xrightarrow[{{H}_{2}}O]{C{{H}_{3}}MgX}\]
done
clear
C)
\[C{{H}_{3}}CH=C{{H}_{2}}\xrightarrow[C{{H}_{3}}COOH]{{{B}_{2}}{{H}_{6}}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\xrightarrow{P/HI}\]
done
clear
View Answer play_arrow
question_answer 96) A compound \[A\to {{C}_{5}}{{H}_{10}}C{{l}_{2}}\] on hydrolysis gives \[{{C}_{5}}{{H}_{10}}O\] which reacts with \[N{{H}_{2}}OH\], forms iodoform, but does not give Fehling test. A is :
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{CH}}\,}}\,\]
done
clear
D)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,H-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 97) In photography, sodium thiosulphate is used as:
A)
complexing agent
done
clear
B)
oxidising agent
done
clear
C)
reducing agent
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 98)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) There is no S?S bond in:
A)
\[{{s}_{2}}O_{4}^{2-}\]
done
clear
B)
\[{{s}_{2}}O_{5}^{2-}\]
done
clear
C)
\[{{s}_{2}}O_{3}^{2-}\]
done
clear
D)
\[{{s}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 100) Which of the following is not a broad spectrum antibiotic?
A)
Tetracycline
done
clear
B)
Chloromycetin
done
clear
C)
Penicillin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 101) Amoeba is a/an:
A)
unicellular animal
done
clear
B)
octacellular animal
done
clear
C)
multicellular animal
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 102) Butterfly belongs to:
A)
Homoptera
done
clear
B)
Procoptera
done
clear
C)
Hemiptera
done
clear
D)
Lepidoptera
done
clear
View Answer play_arrow
question_answer 103) Coverstone of theory of Darwin was :
A)
natural selection
done
clear
B)
inheritance of acquired characters
done
clear
C)
omnis cellula e cellula
done
clear
D)
higher productivity
done
clear
View Answer play_arrow
question_answer 104) Enzyme present in saliva is:
A)
maltase
done
clear
B)
ptyalin
done
clear
C)
sucrase
done
clear
D)
invertase
done
clear
View Answer play_arrow
question_answer 105) The pH of blood is :
A)
between 7-8
done
clear
B)
between 2-4
done
clear
C)
between 12-14
done
clear
D)
between 2-5
done
clear
View Answer play_arrow
question_answer 106) Fungi in a forest ecosystem is :
A)
producer
done
clear
B)
decomposer
done
clear
C)
top consumer
done
clear
D)
autotroph
done
clear
View Answer play_arrow
question_answer 107) Energy enters in a food chain through:
A)
producers
done
clear
B)
primary consumers
done
clear
C)
secondary consumers
done
clear
D)
tertiary consumers
done
clear
View Answer play_arrow
question_answer 108) Cyanobacteria is an:
A)
algae having blue-green pigment
done
clear
B)
algae having red pigment
done
clear
C)
algae having brown pigment
done
clear
D)
algae having yellow-brown pigment
done
clear
View Answer play_arrow
question_answer 109) Cu is present in :
A)
plasmalemma
done
clear
B)
plastoquinone
done
clear
C)
plastocyanin
done
clear
D)
ferredoxin
done
clear
View Answer play_arrow
question_answer 110) In which of the following, oxygen does not evolve during photosynthesis?
A)
Photosynthetic red algae
done
clear
B)
Photosynthetic green algae
done
clear
C)
Photosynthetic blue-green algae
done
clear
D)
Photosynthetic bacteria
done
clear
View Answer play_arrow
question_answer 111) The largest phylum in respect of number of species is :
A)
Coelenterata
done
clear
B)
Arthropoda
done
clear
C)
Protozoa
done
clear
D)
Porifera
done
clear
View Answer play_arrow
question_answer 112) Chronical disturbance in hormone secretion of thyroid gland causes :
A)
goitre
done
clear
B)
diabetes
done
clear
C)
Addisons disease
done
clear
D)
colourblindness
done
clear
View Answer play_arrow
question_answer 113) The formation of egg and sperm is affected by:
A)
LH
done
clear
B)
MH
done
clear
C)
TSH
done
clear
D)
( d) FSH
done
clear
View Answer play_arrow
question_answer 114) ACTH is secreted by :
A)
thyroid gland
done
clear
B)
thymus gland
done
clear
C)
pituitary gland
done
clear
D)
isletsof Langerhans
done
clear
View Answer play_arrow
question_answer 115) Juvenile hormone is secreted by :
A)
thyroid gland
done
clear
B)
thymus gland
done
clear
C)
adrenal gland
done
clear
D)
corpora allata
done
clear
View Answer play_arrow
question_answer 116) Enzymes of electron transport system are present in :
A)
inner mitochondrial membrane
done
clear
B)
matrix
done
clear
C)
intermembranous space
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 117) Ozone hole results in :
A)
UV radiation reaches the earth
done
clear
B)
cataract
done
clear
C)
increase in skin cancer
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 118) Green-house effect is due to the increased concentration of:
A)
\[C{{O}_{2}}\]
done
clear
B)
Ne
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 119) Soil erosion is prevented by :
A)
deforestation
done
clear
B)
afforestation
done
clear
C)
reduction of CFCs production
done
clear
D)
use of CNG in all transports
done
clear
View Answer play_arrow
question_answer 120) Humus is present in :
A)
horizon-A
done
clear
B)
horizon-0
done
clear
C)
horizon-B
done
clear
D)
horizon-C
done
clear
View Answer play_arrow
question_answer 121) Which of the following metal is present in vitamin \[{{B}_{12}}\]?
A)
Cobalt
done
clear
B)
Copper
done
clear
C)
Zinc
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 122) Primary blood cells are formed in :
A)
plasma
done
clear
B)
bone marrow
done
clear
C)
liver
done
clear
D)
spleen
done
clear
View Answer play_arrow
question_answer 123) Secondary host of Schistosoma is:
A)
Hydra
done
clear
B)
Euglena
done
clear
C)
Snail
done
clear
D)
Pheretima
done
clear
View Answer play_arrow
question_answer 124) Nerve cells are the part of:
A)
epithelial tissue
done
clear
B)
connective tissue
done
clear
C)
muscles tissue
done
clear
D)
nervous tissue
done
clear
View Answer play_arrow
question_answer 125) Sertoli cells help in :
A)
maturation of eggs
done
clear
B)
maturation of sperms
done
clear
C)
enzyme production
done
clear
D)
ovulation
done
clear
View Answer play_arrow
question_answer 126) Opium is extracted from :
A)
Atropa belladona
done
clear
B)
Papaver somniferum
done
clear
C)
Vinca rosea
done
clear
D)
Azadirachta indica
done
clear
View Answer play_arrow
question_answer 127) Quinine is obtained from :
A)
bark of Cinchona
done
clear
B)
root of Cinchona
done
clear
C)
wood of Cinchona
done
clear
D)
leaves of Cinchona
done
clear
View Answer play_arrow
question_answer 128) Angiosperms differ from gymnosperms in :
A)
seeds
done
clear
B)
fruits
done
clear
C)
male gametophyte
done
clear
D)
female gametophyte
done
clear
View Answer play_arrow
question_answer 129) When Ronald Ross found malaria parasite infection, in mosquito?
A)
1897
done
clear
B)
1850
done
clear
C)
1835
done
clear
D)
1859
done
clear
View Answer play_arrow
question_answer 130) Genes are present on :
A)
chromosomes
done
clear
B)
lamellae
done
clear
C)
plasma membrane
done
clear
D)
mesosomes
done
clear
View Answer play_arrow
question_answer 131) In the process of photosynthesis :
A)
\[{{O}_{2}}\] is taken and \[C{{O}_{2}}\] is evolved
done
clear
B)
\[{{O}_{2}}\] is taken but \[C{{O}_{2}}\] is not evolved
done
clear
C)
\[C{{O}_{2}}\] is taken and \[{{O}_{2}}\] is evolved
done
clear
D)
\[C{{O}_{2}}\] is taken and \[N{{O}_{2}}\] is evolved
done
clear
View Answer play_arrow
question_answer 132) Bacteria do not have :
A)
ribosome
done
clear
B)
protein synthesizing apparatus
done
clear
C)
mitochondria
done
clear
D)
cell wall
done
clear
View Answer play_arrow
question_answer 133) Which of the following plant hormone if extracted from fungus?
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
Cytokinin
done
clear
D)
2, 4-D
done
clear
View Answer play_arrow
question_answer 134) Highest concentration of auxin is found in :
A)
root and shoot tips
done
clear
B)
leaves and fruits
done
clear
C)
midstem portion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 135) Ethylene is used for :
A)
decrease the senescence
done
clear
B)
increase the height of stem
done
clear
C)
ripening of fruits
done
clear
D)
prevention of leaf fall
done
clear
View Answer play_arrow
question_answer 136) Meristematic tissues are:
A)
premature having ability of division
done
clear
B)
mature does not have ability of division
done
clear
C)
premature not having ability of division
done
clear
D)
complex differentiating in xylem, phloem and cambium
done
clear
View Answer play_arrow
question_answer 137) The function of polymerase chain reaction (PCR) is :
A)
translation
done
clear
B)
transduction
done
clear
C)
DNA amplification
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 138) A codon is made up of:
A)
single nucleotide
done
clear
B)
two nucleotides
done
clear
C)
three nucleotides
done
clear
D)
four nucleotides
done
clear
View Answer play_arrow
question_answer 139) Protein in silk thread is :
A)
fibroin
done
clear
B)
keratin
done
clear
C)
albumin
done
clear
D)
globulin
done
clear
View Answer play_arrow
question_answer 140) Histamine is secreted by:
A)
goblet cell
done
clear
B)
nerve cell
done
clear
C)
Kupffer cell
done
clear
D)
mast cell
done
clear
View Answer play_arrow
question_answer 141) Which of the following hormones are secreted by a pancreas?
A)
Insulin and glucagon
done
clear
B)
Epinephrin and nor-epinephrin
done
clear
C)
Thyroxine and melanin
done
clear
D)
Lactocin and oxytocin
done
clear
View Answer play_arrow
question_answer 142) Starfish belongs to phylum :
A)
Porifera
done
clear
B)
Coelenterata
done
clear
C)
Echinodermata
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 143) Yeast belongs to :
A)
Zygomycetes
done
clear
B)
Basidiomycetes
done
clear
C)
Ascomycetes
done
clear
D)
Phycomycetes
done
clear
View Answer play_arrow
question_answer 144) Mycorrhiza helps in :
A)
nutrition uptaking
done
clear
B)
food manufacturing
done
clear
C)
disease resistance
done
clear
D)
disease prevention
done
clear
View Answer play_arrow
question_answer 145) The conversion of \[N{{O}_{3}}\] to \[{{N}_{2}}\] is called :
A)
nitrification
done
clear
B)
denitrification
done
clear
C)
ammonification
done
clear
D)
nitrogen fixation
done
clear
View Answer play_arrow
question_answer 146) Luscuta is a :
A)
parasitic plant
done
clear
B)
symbiotic plant
done
clear
C)
predator
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 147) Binary fission is a mode of:
A)
micropropagation
done
clear
B)
vegetative propagation
done
clear
C)
macropropagation
done
clear
D)
sexual reproduction
done
clear
View Answer play_arrow
question_answer 148) Humidity in atmosphere decreases rate of:
A)
transpiration
done
clear
B)
photosynthesis
done
clear
C)
glycolysis
done
clear
D)
growth
done
clear
View Answer play_arrow
question_answer 149) In osmosis, there is movement of:
A)
solute only
done
clear
B)
solvent only
done
clear
C)
both [a] and [b]
done
clear
D)
neither solute nor solvent
done
clear
View Answer play_arrow
question_answer 150) Jumping genes in maize were discovered by :
A)
Hugo de Vries
done
clear
B)
T. H. Morgan
done
clear
C)
Barbara McClintock
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 151) Sea horse belongs to :
A)
Mammals
done
clear
B)
Amphibia
done
clear
C)
Aves
done
clear
D)
Pisces
done
clear
View Answer play_arrow
question_answer 152) Kupffer cells are present in :
A)
liver
done
clear
B)
pancreas
done
clear
C)
small intestine
done
clear
D)
large intestine
done
clear
View Answer play_arrow
question_answer 153) Ontogeny repeats phylogeny is a part of:
A)
Recapitulation theory
done
clear
B)
Darwins evolution theory
done
clear
C)
Lamarcks evolution theory
done
clear
D)
Weismanns theory
done
clear
View Answer play_arrow
question_answer 154) Who gave theory about embryonic evolution?
A)
Darwin
done
clear
B)
Lamarck
done
clear
C)
Haeckel
done
clear
D)
Dabzansky
done
clear
View Answer play_arrow
question_answer 155) Primitive man was originated during :
A)
Miocene
done
clear
B)
Holocene
done
clear
C)
Pleistocene
done
clear
D)
Pliocene
done
clear
View Answer play_arrow
question_answer 156) Universal blood recipient is :
A)
blood group-O
done
clear
B)
blood group-AB
done
clear
C)
blood group-A
done
clear
D)
blood group-B
done
clear
View Answer play_arrow
question_answer 157) Plant like nutrition is present in :
A)
Amoeba
done
clear
B)
Paramedum
done
clear
C)
Euglena
done
clear
D)
Plasmodium
done
clear
View Answer play_arrow
question_answer 158) Which of the following is connecting link between reptiles and birds?
A)
Archaeopteryx
done
clear
B)
Euglena
done
clear
C)
Neopilina
done
clear
D)
Latimeria
done
clear
View Answer play_arrow
question_answer 159) Which of the following connects glycolysis to Krebs cycle?
A)
Acetyl Co-A
done
clear
B)
Ribozyme
done
clear
C)
Cytochrome oxidase
done
clear
D)
N-acetyl glucosamine
done
clear
View Answer play_arrow
question_answer 160) Pyruvic acid is the end product of:
A)
Krebs cycle
done
clear
B)
electron transport system
done
clear
C)
photosynthesis
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 161) Which of the following law was discovered first by Mendel?
A)
Law of dominance
done
clear
B)
Law of segregation
done
clear
C)
Law of independent assortment
done
clear
D)
Law of sex determination
done
clear
View Answer play_arrow
question_answer 162) Which of the following accepts terminal electron?
A)
Molecular\[{{O}_{2}}\]
done
clear
B)
Molecular \[{{H}_{2}}\]
done
clear
C)
Molecular \[C{{O}_{2}}\]
done
clear
D)
\[NADP{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 163) Which of the following is a viral disease?
A)
Typhoid
done
clear
B)
Polio
done
clear
C)
T.B.
done
clear
D)
Leprosy
done
clear
View Answer play_arrow
question_answer 164) Which one is a sex linked disease ?
A)
Colourblindness
done
clear
B)
Haemophilia
done
clear
C)
Syphilis
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 165) Metamorphosis in frog is hastened by :
A)
thyroxine
done
clear
B)
insulin
done
clear
C)
glucagon
done
clear
D)
adrenalin
done
clear
View Answer play_arrow
question_answer 166) Which have no specific organ for respiration but respire?
A)
Rabbit
done
clear
B)
Cockroach
done
clear
C)
Earthworm
done
clear
D)
Frog
done
clear
View Answer play_arrow
question_answer 167) The study of reptiles is called :
A)
Herpetology
done
clear
B)
Dermatology
done
clear
C)
Angiology
done
clear
D)
Parazoology
done
clear
View Answer play_arrow
question_answer 168) Tendons and ligaments are :
A)
epithelial tissue
done
clear
B)
fibrous connective tissue
done
clear
C)
nerve tissue
done
clear
D)
muscular tissue
done
clear
View Answer play_arrow
question_answer 169) Mushroom belongs to:
A)
Ascomycetes
done
clear
B)
Basidiomycetes
done
clear
C)
Phycomycetes
done
clear
D)
Zygomycetes
done
clear
View Answer play_arrow
question_answer 170) The wall of bacteria consists of :
A)
N-acetyl glucosamine
done
clear
B)
N-acetyl muramic acid
done
clear
C)
both [a] and [b]
done
clear
D)
cellulose
done
clear
View Answer play_arrow
question_answer 171) The movement of pollen tube is called :
A)
chemotropism
done
clear
B)
thermotaxis
done
clear
C)
thermonastic
done
clear
D)
hydrotropism
done
clear
View Answer play_arrow
question_answer 172) What will be the gametic chromosome number of a cell, if somatic cell have 40 chromosomes?
A)
10
done
clear
B)
20
done
clear
C)
30
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 173) Who coined the term cell?
A)
Purkinje
done
clear
B)
Robert Brown
done
clear
C)
Robert Hooke
done
clear
D)
Hugo von Mohl
done
clear
View Answer play_arrow
question_answer 174) Which of the following animals is mostly used in genetics experiments ?
A)
Butterfly
done
clear
B)
Fruitfly
done
clear
C)
Housefly
done
clear
D)
Dragonfly
done
clear
View Answer play_arrow
question_answer 175) In which of the following stage chromosomes are arranged at equatorial plate?
A)
Anaphase
done
clear
B)
Metaphase
done
clear
C)
Prophase
done
clear
D)
Telophase
done
clear
View Answer play_arrow
question_answer 176) Which of the following is called as Age of Reptiles?
A)
Coenozoic era
done
clear
B)
Azoic era
done
clear
C)
Mesozoic era
done
clear
D)
Precambrian era
done
clear
View Answer play_arrow
question_answer 177) Who first conducted experiment on evolution to prove biochemical origin of life?
A)
Miller and Urey
done
clear
B)
Darwin
done
clear
C)
Lamarck
done
clear
D)
Weismann
done
clear
View Answer play_arrow
question_answer 178) Maturation of sperm before penetration is called:
A)
spermatogenesis
done
clear
B)
spermiogenesis
done
clear
C)
capacitation
done
clear
D)
spermatid
done
clear
View Answer play_arrow
question_answer 179) Echolocation is. found in :
A)
bat
done
clear
B)
cat
done
clear
C)
dog
done
clear
D)
horse
done
clear
View Answer play_arrow
question_answer 180) Life span of RBC is :
A)
50 days
done
clear
B)
70 days
done
clear
C)
120 days
done
clear
D)
220 days
done
clear
View Answer play_arrow
question_answer 181) During mitosis number of chromosomes gets :
A)
change
done
clear
B)
no change
done
clear
C)
may be change if cell is mature
done
clear
D)
may be change if cell is immature
done
clear
View Answer play_arrow
question_answer 182) Which of the following enzyme is used in DNA multiplication?
A)
RNA pplymerase
done
clear
B)
DNA endonuclease
done
clear
C)
Exonuclease
done
clear
D)
DNA polymerase
done
clear
View Answer play_arrow
question_answer 183) The back bone of RNA consists of which of the following sugar ?
A)
Deoxyribose
done
clear
B)
Ribose
done
clear
C)
Sucrose
done
clear
D)
Maltose
done
clear
View Answer play_arrow
question_answer 184) Phytotron is:
A)
a controlled condition chamber for tissue culture
done
clear
B)
leaf culture process
done
clear
C)
special culture of plants
done
clear
D)
root culture process
done
clear
View Answer play_arrow
question_answer 185) Phloem conducts food by :
A)
perforated sieve plates
done
clear
B)
bast fibres
done
clear
C)
xylem parenchyma
done
clear
D)
xylem fibres
done
clear
View Answer play_arrow
question_answer 186) The purpose of crop rotation is to :
A)
increase the fertility of soil
done
clear
B)
decrease the fertility of soil
done
clear
C)
prevent soil erosin
done
clear
D)
prevent water erosion
done
clear
View Answer play_arrow
question_answer 187) Which of the following is a nitrogen fixing organism?
A)
BGA
done
clear
B)
Rhizobium
done
clear
C)
Both [a] and [b]
done
clear
D)
Aspergillus
done
clear
View Answer play_arrow
question_answer 188) To maintain constant body temperature is called:
A)
homeothermic
done
clear
B)
poikilothermic
done
clear
C)
homozygous
done
clear
D)
heterozygous
done
clear
View Answer play_arrow
question_answer 189) African sleeping sickness is caused by :
A)
Trypanosoma
done
clear
B)
Leishmania
done
clear
C)
Latimeria
done
clear
D)
Plasmodium
done
clear
View Answer play_arrow
question_answer 190) Natural pearl belongs to :
A)
Arthropoda
done
clear
B)
Protozoa
done
clear
C)
Mollusca
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 191) During blood clotting which of the following is used?
A)
Co
done
clear
B)
\[C{{a}^{2+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 192) The chromosome showing L-shaped structure by the presence of centromere is termed as:
A)
acentric
done
clear
B)
metacentric
done
clear
C)
sub-metacentric
done
clear
D)
telocentric
done
clear
View Answer play_arrow
question_answer 193) The infected stage of malarial parasite is :
A)
sporozoites stage
done
clear
B)
schizozoites stage
done
clear
C)
merozoites stage
done
clear
D)
metacryptozoites stage
done
clear
View Answer play_arrow
question_answer 194) Haemoglobin contains:
A)
\[F{{e}^{2+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[N{{a}^{2+}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 195) Chromosomes can be seen best during :
A)
prophase
done
clear
B)
metaphase
done
clear
C)
anaphase
done
clear
D)
telophase
done
clear
View Answer play_arrow
question_answer 196) Which of the following is concerned with \[C{{O}_{2}}\]fixation?
A)
Krebs cycle
done
clear
B)
Calvin cycle
done
clear
C)
Omithine cycle
done
clear
D)
Glycolysis
done
clear
View Answer play_arrow
question_answer 197) In photosynthesis there is:
A)
reduction of \[{{H}_{2}}O\]
done
clear
B)
oxidation of \[{{H}_{2}}O\]
done
clear
C)
oxidation of \[C{{O}_{2}}\]
done
clear
D)
oxidation of \[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 198) Pigment present in cyanobacteria is :
A)
r-phycocyanin
done
clear
B)
r-phycoerythrin
done
clear
C)
c-phycocyanin
done
clear
D)
anthocyanin
done
clear
View Answer play_arrow
question_answer 199) Multiplication of DNA is called :
A)
transcription
done
clear
B)
translation
done
clear
C)
replication
done
clear
D)
transduction
done
clear
View Answer play_arrow
question_answer 200) ELISA test is used for detection of:
A)
antibodies
done
clear
B)
viral disease
done
clear
C)
AIDS
done
clear
D)
all of these
done
clear
View Answer play_arrow
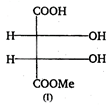
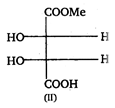 (I) and (II) are:
(I) and (II) are: