question_answer 1) The radius of a soap bubble is R and surface tension is T, keeping the temperature constant, the extra energy needed to double the radius of the soap bubble by blowing, will be:
A)
\[32\pi {{R}^{2}}T\]
done
clear
B)
\[24\pi {{R}^{2}}T\]
done
clear
C)
\[16\pi {{R}^{2}}T\]
done
clear
D)
\[8\pi {{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 2) An ideal gas at \[27{}^\circ C\] is compressed adiabatically to \[\frac{8}{27}\]of its original volume. If \[\gamma \]= 5/3, then the rise in temperature is :
A)
375 K
done
clear
B)
450 K
done
clear
C)
225 K
done
clear
D)
405 K
done
clear
View Answer play_arrow
question_answer 3) A uniform chain of length L and mass M is lying on a smooth table and one third of its length is hanging vertically downward over the edge of table, if g is acceleration due to gravity, then the work done required to pull the hanging part on to the table is :
A)
MgL
done
clear
B)
MgL/3
done
clear
C)
MgL
done
clear
D)
MgL/18
done
clear
View Answer play_arrow
question_answer 4) Two planets have the same average density but their radii are \[{{R}_{1}}\]and \[{{R}_{2}}.\] If acceleration due to gravity on these planets be \[{{g}_{1}}\] and \[{{g}_{2}},\] then :
A)
\[\frac{g1}{g2}=\frac{R_{1}^{3}}{R_{2}^{3}}\]
done
clear
B)
\[\frac{g1}{g2}=\frac{R_{1}^{2}}{R_{2}^{2}}\]
done
clear
C)
\[\frac{g1}{g2}=\frac{{{R}_{2}}}{{{R}_{1}}}\]
done
clear
D)
\[\frac{g1}{g2}=\frac{{{R}_{1}}}{{{R}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 5) Two metal pieces having a potential difference of 800V are 0.02 m apart horizontally. A particle of mass\[1.96\times {{10}^{-15}}\,kg\]is suspended equilibrium between the plates. If e is the elementary charge, then charge on the particle is :
A)
8e
done
clear
B)
6e
done
clear
C)
3e
done
clear
D)
e
done
clear
View Answer play_arrow
question_answer 6) \[\frac{1}{2}\]mole of He is contained in a container at S.T.P. The heat energy needed to double the pressure of the gas, keeping the volume constant, (heat capacity of the gas is 3J/gmK) is :
A)
409.5 J
done
clear
B)
819 J
done
clear
C)
1638 J
done
clear
D)
3276 J
done
clear
View Answer play_arrow
question_answer 7) A sphere of 3 cm radius acts like a black body. If it is an equilibrium with its surroundings and absorb 30kW of energy radiated to it from the surroundings. Then the temperature of the sphere will be :
A)
816
done
clear
B)
916
done
clear
C)
1616
done
clear
D)
2615
done
clear
View Answer play_arrow
question_answer 8) The maximum velocity of an electron emitted by light of wavelength X incident on the surface of a metal of work function \[\theta \], is :
A)
\[{{\left[ \frac{2(h\lambda -\theta )}{m} \right]}^{{1}/{2}\;}}\]
done
clear
B)
\[{{\left[ \frac{2(hc-\lambda \phi )}{m\lambda } \right]}^{{1}/{2}\;}}\]
done
clear
C)
\[\left[ \frac{2(hc-\lambda \phi )}{m} \right]\]
done
clear
D)
\[{{\left[ \frac{2(hc-\lambda \phi )}{m\lambda } \right]}^{{1}/{2}\;}}\]
done
clear
View Answer play_arrow
question_answer 9) The average distance between the earth and moon is \[38.6\times {{10}^{7}}\]km. The minimum separation between the two points on the surface of the moon that can be resolved by a telescope whose objective lens has a diameter of 5 m with \[\lambda \] = 6000 \[\overset{0}{\mathop{A}}\,\] is :
A)
56.51 m
done
clear
B)
11.30m
done
clear
C)
28.25m
done
clear
D)
5.65 m
done
clear
View Answer play_arrow
question_answer 10) A transverse progressive wave on a stretched string has a velocity of 10 m/s and a frequency of 100 Hz. The phase difference between two particles of the string which are 2.5 cm apart will be :
A)
\[\frac{\pi }{2}\]
done
clear
B)
\[\frac{3\pi }{8}\]
done
clear
C)
\[\pi /4\]
done
clear
D)
\[\pi /8\]
done
clear
View Answer play_arrow
question_answer 11) Water waves are :
A)
transverse
done
clear
B)
longitudinal
done
clear
C)
both longitudinal and transverse
done
clear
D)
neither longitudinal nor transverse
done
clear
View Answer play_arrow
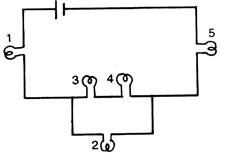
question_answer 12)
All bulbs in the figure are identical which bulb lights more brightly?
A)
1 and 2
done
clear
B)
3 and 4
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 13) A capacitor is a perfect insulator for :
A)
A.C
done
clear
B)
D.C
done
clear
C)
both A.C and D.C
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 14) Just as electricity is supplied at 220 volts for domestic use in India, it is supplied at110 volts in U.S.A. If the resistance of 60 W bulb for use in India is R , that of 60 W bulb for use in U.S.A., will be :
A)
2R
done
clear
B)
R
done
clear
C)
RJ2
done
clear
D)
R/4
done
clear
View Answer play_arrow
question_answer 15) A proton moving with a constant velocity passes through a region of space without any change in its velocity. If E and B represent the electric and magnetic fields respectively, then this region of space may have :
A)
E = 0, B = 0
done
clear
B)
\[E\ne 0,B1+0\]
done
clear
C)
\[E=0,B\ne 0\]
done
clear
D)
and both
done
clear
View Answer play_arrow
question_answer 16) The decimal number 53 is equal to the binary number:
A)
11 01 01
done
clear
B)
10 11 01
done
clear
C)
10 10 10
done
clear
D)
11 11 11
done
clear
View Answer play_arrow
question_answer 17) \[A6\mu F\]capacitor is charged from 10 volts to 20 volts. Increase in energy will be :
A)
\[9\times {{10}^{-6}}J\]
done
clear
B)
\[4.5\times {{10}^{-4}}J\]
done
clear
C)
\[9\times {{10}^{-4}}J\]
done
clear
D)
\[19\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
question_answer 18) An electric bulb of 100 W is connected to a supply of electricity of 200V. Resistance of the filament is :
A)
242 \[\Omega \]
done
clear
B)
22000 \[\Omega \]
done
clear
C)
100\[\Omega \]
done
clear
D)
484 \[\Omega \]
done
clear
View Answer play_arrow
question_answer 19) A body of mass 40 g is moving with constant velocity of 2 cm/sec on a horizontal frictionless table. The force on the table is :
A)
160 dyne
done
clear
B)
39200 dyne
done
clear
C)
80 dyne
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 20) A cup of tea cools from \[80{}^\circ C\] to \[60{}^\circ C\] in one minute. The ambient temperature is \[30{}^\circ C\]. In cooling from \[60{}^\circ C\] to \[50{}^\circ C\] it will take :
A)
50 sec
done
clear
B)
90 sec
done
clear
C)
60 sec
done
clear
D)
48 sec
done
clear
View Answer play_arrow
question_answer 21) The magnifying power of simple microscope can be increased, if we use an eye piece of:
A)
smaller diameter
done
clear
B)
higher diameter
done
clear
C)
smaller focal length
done
clear
D)
higher focal length
done
clear
View Answer play_arrow
question_answer 22) The number of photoelectrons emitted from the surface of a metal increases on increasing of the incident photons:
A)
velocity
done
clear
B)
wavelength
done
clear
C)
frequency
done
clear
D)
number
done
clear
View Answer play_arrow
question_answer 23) When two ends of a rod wrapped with cotton are maintained at different temperature and after some time every point of the rod attains a constant temperature, then
A)
each point of rod is giving heat to its neighbour at the same rate which it is receiving heat
done
clear
B)
rod is bad conductor of heat
done
clear
C)
heat is being radiated from each point of rod
done
clear
D)
conduction of heat at different points of the rod stops because the temperature is not increasing
done
clear
View Answer play_arrow
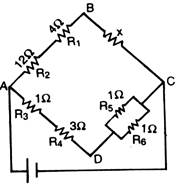
question_answer 24)
In the circuit shown in the adjoining figure the current between B and D is zero, the unknown resistance is of:
A)
3\[\Omega \]
done
clear
B)
2\[\Omega \]
done
clear
C)
4\[\Omega \]
done
clear
D)
emf of a cell is required to final the value of X.
done
clear
View Answer play_arrow
question_answer 25) A conductor has \[14.4\times {{10}^{-19}}C\] positive charge. The conductor has :
A)
excess of 27 electrons
done
clear
B)
excess of 9 electrons
done
clear
C)
short of 27 electrons
done
clear
D)
short of 9 electrons
done
clear
View Answer play_arrow
question_answer 26) In the position of minimum deviation when a ray of yellow light passes through the prism, then its angle of incidence is:
A)
greater than the emergent angle
done
clear
B)
less than the emergent angle
done
clear
C)
equa1 to the emergent angle
done
clear
D)
sum of angle of incidence and emergent angle is 90°
done
clear
View Answer play_arrow
question_answer 27) The vertical extension in a light spring by a weight of 1 kg suspended from the wire is 9.8 cm. The period of oscillation is :
A)
200 \[\pi \]sec
done
clear
B)
2\[\pi \]/10 sec
done
clear
C)
2\[\pi \] sec
done
clear
D)
4\[\pi \] sec
done
clear
View Answer play_arrow
question_answer 28) A meter of resistance 5 ohm fitted with a shunt resistance of 1 ohm, is in a circuit in which the current is 0.09 A. The combined resistance of the meter and the shunt is :
A)
\[\frac{5}{6}\Omega \]
done
clear
B)
\[\frac{5}{6}\Omega \]
done
clear
C)
\[2\Omega \]
done
clear
D)
\[6\Omega \]
done
clear
View Answer play_arrow
question_answer 29) A source and an observer moves away from each other, with a velocity of 15 m/s with respect to ground. If observer finds the frequency of sound coming from source as 1950 Hz. Then actual frequency of source will be (velocity of sound = 340 m/s):
A)
1785 Hz
done
clear
B)
1968 Hz
done
clear
C)
2130 Hz
done
clear
D)
2148 Hz
done
clear
View Answer play_arrow
question_answer 30) If the coefficient of friction of a plane inclined at 45°, is 0.5, then acceleration of a body sliding freely on it is:
A)
\[\frac{9.8}{2\sqrt{2}}m/{{s}^{2}}\]
done
clear
B)
\[\frac{9.8}{\sqrt{2}}m/{{s}^{2}}\]
done
clear
C)
\[9.8m/{{s}^{2}}\]
done
clear
D)
\[4.9m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 31) A body of mass 300 kg is moved up by an elevator from a mine of depth 20 m to a height of 20 m in 1 min. The power of the elevator is :
A)
19.6 kW
done
clear
B)
196 kW
done
clear
C)
0.196 kW
done
clear
D)
1.96 kW
done
clear
View Answer play_arrow
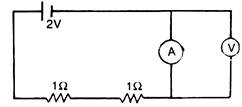
question_answer 32)
In the circuit given below, A and V are ideal ammeter and voltmeter respectively. Reading of the voltmeter will be:
A)
1 V
done
clear
B)
0.5 V
done
clear
C)
2 V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 33) A parallel plate capacitor with air as medium between the plates has a capacitance of 10\[\mu F\]. The area of capacitor is divided in two equal halves and filled with two media having dielectric constants \[{{k}_{1}}=2\] and \[{{k}_{2}}=4\]The capacitance of the system will now be :
A)
40\[\mu F\]
done
clear
B)
30\[\mu F\]
done
clear
C)
10\[\mu F\]
done
clear
D)
\[\frac{20}{3}\mu F\]
done
clear
View Answer play_arrow
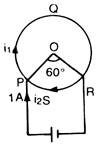
question_answer 34)
What is the current in the circuit shown below?
A)
1A
done
clear
B)
zero A
done
clear
C)
\[{{10}^{-2}}A\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 35) The de-Broglie wavelength of a 1 kg object moving with speed of 1 m/s is :
A)
\[6\times {{10}^{-10}}m\]
done
clear
B)
\[6.6\times {{10}^{-10}}m\]
done
clear
C)
\[6\times {{10}^{-34}}m\]
done
clear
D)
\[6.6\times {{10}^{-34}}m\]
done
clear
View Answer play_arrow
question_answer 36) A ray of monochromatic light is incident on one refracting face of a prism of angle \[75{}^\circ \]. It passes through the prism and is incident on the other face at the critical angle. If the refractive index of the material of prism is \[\sqrt{2,}\], the angle of incidence on the first face of the prism is :
A)
\[0{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 37) As a result of radioactive decay, \[_{92}{{\mathsf{U}}^{238}}\] is converted into\[_{91}P{{a}^{234}}\]. The particles emitted during this decay are:
A)
\[1\alpha ,1\beta \]
done
clear
B)
\[2\beta {{,}_{1}}{{H}^{1}}\]
done
clear
C)
\[_{1}{{H}^{1}}\]
done
clear
D)
\[_{1}{{H}^{1}},2\alpha \]
done
clear
View Answer play_arrow
question_answer 38) The half-life of radioactive radon is 3.8days. The time at the end of which \[\frac{1}{20}\]th of the radon sample will remain un decayed is: \[\left( {{\log }_{10}}e=0.4343 \right)\]
A)
16.5 days
done
clear
B)
3.8 days
done
clear
C)
33 days
done
clear
D)
76 days
done
clear
View Answer play_arrow
question_answer 39) The charge on two particles A and B at rest is q on each particle. The direction of motion of a charged particles C placed at point on the straight line joining the two charged, depends on :
A)
the charge on C and also on its position
done
clear
B)
only on the position of charge C
done
clear
C)
only on the magnitude of charge on C
done
clear
D)
only on the magnitudes of the charges of A and B
done
clear
View Answer play_arrow
question_answer 40) The potential gradient at which the dielectric of a condenser just gets punctured is called:
A)
dielectric number
done
clear
B)
dielectric resistance
done
clear
C)
dielectric strength
done
clear
D)
dielectric constant
done
clear
View Answer play_arrow
question_answer 41) The power of a thin convex lens (\[_{a}{{n}_{g}}\] = 1.5) is + 5D. When it is placed in a liquid of refractive index\[_{a}nl,\], then it behave as a concave lens of focal length 100 cm. The refractive index of the liquid \[_{a}nl,\] will be:
A)
5/4
done
clear
B)
\[\sqrt{3}\]
done
clear
C)
4/3
done
clear
D)
5/3
done
clear
View Answer play_arrow
question_answer 42) A bus accelerates uniformally from rest and acquires a speed of 72 km/hr in 10 sec. The acceleration of the bus is :
A)
\[2\text{ }m/{{s}^{2}}\]
done
clear
B)
\[1\text{ }m/{{s}^{2}}\]
done
clear
C)
\[4\text{ }m/{{s}^{2}}\]
done
clear
D)
\[20\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 43) In a nuclear reactor 0.01 mg of a nuclear material is totally converted into energy in one sec. Then the power of reactor will be :
A)
900 MW
done
clear
B)
450 W
done
clear
C)
200 MW
done
clear
D)
500 W
done
clear
View Answer play_arrow
question_answer 44) A spherical conductor of radius r placed in air, is given a charge Q and potentialat the surface of a conductor is V, then the potential at a point inside the conductor and at a distance r/2 from its centre will be:
A)
8 V
done
clear
B)
4 V
done
clear
C)
2 V
done
clear
D)
1 V
done
clear
View Answer play_arrow
question_answer 45) The magnetic moment of a short magnet is \[8\text{ }A{{m}^{2}}.\] The magnetic induction at appoint 20 cm away from its mid-point on (i) a axial point (ii) equatorial point respectively, will be :
A)
\[2\times {{10}^{-4}}T\,and{{10}^{-4}}T\]
done
clear
B)
\[3\times {{10}^{-4}}T\,and2\times {{10}^{-4}}T\]
done
clear
C)
\[4\times {{10}^{-4}}T\,and3\times {{10}^{-4}}T\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 46) Two strings of the same material and of same length have their tension in the ratio 4 :1 and their radii in the ratio 2:1. Then the ratio of their fundamental frequencies will be:
A)
1
done
clear
B)
0.5
done
clear
C)
4
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 47) A voltmeter has a range is 0 - V with, a series resistance R and with a series resistance 2R, the range is 0-V. The relation between V and V will be :
A)
\[V<2V\]
done
clear
B)
\[V>>V\]
done
clear
C)
V > 2V
done
clear
D)
V = 2V
done
clear
View Answer play_arrow
question_answer 48) A galvanometer has a resistance G and current \[{{i}_{a}}\] flowing in galvanometer, produces full scale deflection. If \[{{S}_{i}}\] is the value of shunt which converts it into an ammeter of range 0 - i and is the value of shunt 0 - 2i. Then the ratio \[{{S}_{1}}/{{S}_{2}}\]will be:
A)
1
done
clear
B)
2
done
clear
C)
\[\frac{1}{2}\left( \frac{i-{{i}_{a}}}{2i-{{i}_{a}}} \right)\]
done
clear
D)
\[\left( \frac{2i-{{i}_{a}}}{i-{{i}_{a}}} \right)\]
done
clear
View Answer play_arrow
question_answer 49) A short magnetic needle is pivoted in a uniform magnetic field of strength 1 tesla, when another magnetic field of strength \[\sqrt{3}\]T is applied to the needle in a perpendicular directions. The needle deflects at an angle \[\theta \], when the value of \[\theta \] is :
A)
\[60{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 50)
A cell is connected between the points A and C of a circular conductor PQRS of centre O with angle \[\angle \]POR= 60°. If \[{{B}_{1}}\]and \[{{B}_{2}}\]are the magnitudes of magnetic fields at O due to the currents in PQR and PSR respectively, then the ratio of \[\frac{{{B}_{1}}}{{{B}_{2}}}\]will be
A)
5:1
done
clear
B)
2:1
done
clear
C)
10 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 51) The total number of unpaired electrons in \[C{{r}^{+3}}\] ion are:
A)
3
done
clear
B)
2
done
clear
C)
4
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 52) Which one of the following electronic configuration have highest second ionisation energy?
A)
\[1{{s}^{2,}}2{{s}^{2}}2{{p}^{4}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{1}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{1}}\]
done
clear
View Answer play_arrow
question_answer 53) Mass of an a-particle is 4.0028 amu, its binding energy per nucleon will be (given, \[{{m}_{p}}=1.0073\] and \[{{m}_{n}}=1.0087\]):
A)
\[2.86MeV\]
done
clear
B)
\[6.79MeV\]
done
clear
C)
\[4.46MeV\]
done
clear
D)
\[8.24MeV\]
done
clear
View Answer play_arrow
question_answer 54) Bond order of \[{{N}_{2}}\] is:
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 55) Which of the following isoelcctronic species \[C{{a}^{+2}},\,C{{l}^{-1}},{{K}^{+}}\] and \[{{S}^{-2}}\] has maximum ionic size?
A)
\[{{K}^{+}}\]
done
clear
B)
\[C{{a}^{+2}}\]
done
clear
C)
\[C{{l}^{-}}\]
done
clear
D)
\[{{S}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 56) Oxidation number of S in \[SO_{4}^{-2}\] is:
A)
\[+4\]
done
clear
B)
\[+6\]
done
clear
C)
\[+5\]
done
clear
D)
\[+8\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following is diamagnetic?
A)
\[{{S}^{-2}}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[N_{2}^{-}\]
done
clear
D)
\[NO\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following is not a Lewis acid?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[Si{{F}_{4}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[FeC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 59) The hybridisation and structure of \[Si{{(C{{H}_{3}})}_{4}}\] is:
A)
sp, linear
done
clear
B)
\[s{{p}^{2}}\] , trigonal planar
done
clear
C)
\[s{{p}^{3}}\] , tetrahedral
done
clear
D)
\[s{{p}^{3}}d\], octahedral
done
clear
View Answer play_arrow
question_answer 60) The number of a-particles and \[\beta \] particles emitted in the reaction \[_{92}{{U}^{238}}{{\xrightarrow{{}}}_{82}}P{{b}^{206}}\] are:
A)
\[6\alpha ,4\beta \]
done
clear
B)
\[8\alpha ,6\beta \]
done
clear
C)
\[4\alpha ,5\beta \]
done
clear
D)
\[6\alpha ,8\beta \]
done
clear
View Answer play_arrow
question_answer 61) Oxidation number of Fe in \[{{K}_{3}}[Fe{{(CN)}_{6}}]\] is:
A)
\[+1\]
done
clear
B)
\[+2\]
done
clear
C)
\[+3\]
done
clear
D)
\[+\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following has highest magnetic moment?
A)
\[C{{r}^{+3}}\]
done
clear
B)
\[{{V}^{+3}}\]
done
clear
C)
\[C{{o}^{+3}}\]
done
clear
D)
\[F{{e}^{+3}}\]
done
clear
View Answer play_arrow
question_answer 63) Which one of die following ores is a chloride?
A)
Zincite
done
clear
B)
Horn silver
done
clear
C)
Bauxite
done
clear
D)
Feldspar
done
clear
View Answer play_arrow
question_answer 64) The product formed in the reaction \[BC{{l}_{3}}+{{H}_{2}}O\xrightarrow{{}}\] products, is :
A)
\[{{B}_{2}}{{H}_{6}}+HCl\]
done
clear
B)
\[{{B}_{2}}{{O}_{3}}+HOCl\]
done
clear
C)
\[{{H}_{3}}B{{O}_{3}}+HCl\]
done
clear
D)
\[B{{H}_{3}}+HCl\]
done
clear
View Answer play_arrow
question_answer 65) Which one of the following is the strongest acid?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[HF\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following indicator is used in the titration of oxalic acid against sodium hydroxide solution?
A)
Fluorescein
done
clear
B)
Methyl red
done
clear
C)
Methyl orange
done
clear
D)
Phenolphthalien
done
clear
View Answer play_arrow
question_answer 67) Glaubers salt is:
A)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
B)
\[N{{a}_{2}}S{{O}_{3}}.2{{H}_{2}}O\]
done
clear
C)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}.5{{H}_{2}}O\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}.10{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 68) When sodium is heated in a current of \[C{{O}_{2}}\] at\[{{260}^{o}}C\], the product obtained is:
A)
sodium for mate
done
clear
B)
sodium oxalate
done
clear
C)
sodium corbonate
done
clear
D)
sodium acetate
done
clear
View Answer play_arrow
question_answer 69) The momentum for the radiation of wavelength 0.33 nm is :
A)
\[3.56\times {{10}^{-16}}\]
done
clear
B)
\[4.32\times {{10}^{22}}\]
done
clear
C)
\[2.01\times {{10}^{-24}}\]
done
clear
D)
\[3.12\times {{10}^{-28}}\]
done
clear
View Answer play_arrow
question_answer 70) When \[KI\] is added in the solution of \[HgC{{l}_{2}}\], the colour of the resultant solution is:
A)
blue
done
clear
B)
red
done
clear
C)
orange
done
clear
D)
scarlet
done
clear
View Answer play_arrow
question_answer 71) Silicon and copper are cooled to a temperature of 300K, then resistivity:
A)
for Cu increases and for Si decreases
done
clear
B)
for Si increases and for Cu decreases
done
clear
C)
decreases for both Si and Cu
done
clear
D)
increases for both Si and Cu
done
clear
View Answer play_arrow
question_answer 72) Total number of a and k bonds in the structure of \[{{P}_{3}}{{O}_{10}}\] are:
A)
\[12\sigma ,6\pi \]
done
clear
B)
\[6\sigma ,10\pi \]
done
clear
C)
\[18\sigma ,6\pi \]
done
clear
D)
\[16\sigma ,4\pi \]
done
clear
View Answer play_arrow
question_answer 73) Which one of the following exhibits geometrical isomerism?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 74) Which of the following ions is most stable?
A)
\[\overset{\oplus }{\mathop{C{{H}_{3}}CHC{{H}_{2}}C{{H}_{3}}}}\,\]
done
clear
B)
\[\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{{{H}_{3}}C-C-\overset{\oplus }{\mathop{C{{H}_{2}}}}\,}}\,}}\,}}\,}}\,\]
done
clear
C)
\[\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{{{H}_{3}}C-C\oplus }}\,}}\,}}\,}}\,\]
done
clear
D)
\[{{H}_{3}}C-C{{H}_{3}}-\overset{\oplus }{\mathop{C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 75) 1-phenyl ethanol can be prepared by the reaction of \[{{C}_{6}}{{H}_{5}}CHO\] with :
A)
\[C{{H}_{3}}I+Mg\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}Br+{{H}_{2}}O\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}Br+Alc.KOH\]
done
clear
D)
\[C{{H}_{3}}Br=A{{l}_{4}}{{C}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) Total number of isomers possible for the compound \[{{C}_{8}}{{H}_{10}}\] are:
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 77) Acetylene on treatment with \[{{H}_{2}}S{{O}_{4}}\] and \[HgS{{O}_{4}}\] produces:
A)
aldehyde
done
clear
B)
ketone
done
clear
C)
alcohol
done
clear
D)
acid
done
clear
View Answer play_arrow
question_answer 78) The strongest of the four acids listed below is:
A)
\[C{{l}_{3}}C-COOH\]
done
clear
B)
\[{{F}_{3}}C-COOH\]
done
clear
C)
\[B{{r}_{3}}C-COOH\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 79) Glucose and cane sugar can be distinguished by:
A)
Molisch test
done
clear
B)
Fehlings solution
done
clear
C)
Baeyers reagent
done
clear
D)
Ammonical \[AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80) Propone and propyne can be distinguished by:
A)
\[B{{r}_{2}}\] in \[CC{{l}_{4}}\]
done
clear
B)
dil \[KMn{{O}_{4}}\]
done
clear
C)
Ammonical \[AgN{{O}_{3}}\]
done
clear
D)
cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 81)
A)
p-bromo aniline
done
clear
B)
p-bromo fluoro benzene
done
clear
C)
2, 4, 6 tribromo fluoro benzene
done
clear
D)
1, 3, 5 tribromo benzene
done
clear
View Answer play_arrow
question_answer 82) Acetaldehyde differ from benzaldehyde and formaldehyde in the reaction with:
A)
\[HCN\]
done
clear
B)
Benedicts solution
done
clear
C)
\[NaOH\]
done
clear
D)
Semicarbazide
done
clear
View Answer play_arrow
question_answer 83) The percentage of ethyl alcohol in rectified sprit is:
A)
\[85.5\]
done
clear
B)
\[75.0\]
done
clear
C)
\[95.6\]
done
clear
D)
\[100\]
done
clear
View Answer play_arrow
question_answer 84) Antibodies are:
A)
enzymes
done
clear
B)
protein
done
clear
C)
carbohydrates
done
clear
D)
hormones
done
clear
View Answer play_arrow
question_answer 85) Salicylic acid on heating with acetic anhydride in presence of cone. \[{{H}_{2}}S{{O}_{3}}\] produces:
A)
resin
done
clear
B)
drug
done
clear
C)
dye
done
clear
D)
explosive
done
clear
View Answer play_arrow
question_answer 86) 5 litres of a gas weighs 14.4 g at NTP. The molecular weight of gas is :
A)
\[64.51\]
done
clear
B)
\[56.42\]
done
clear
C)
\[52.42\]
done
clear
D)
\[61.40\]
done
clear
View Answer play_arrow
question_answer 87) Weight of \[KMn{{O}_{4}}\] required to prepare 250 ml of its \[\frac{N}{10}\]. solution if equivalent weight of \[KMn{{O}_{4}}\]is 31.6, will be:
A)
\[0.64g\]
done
clear
B)
\[1.24g\]
done
clear
C)
\[1.78g\]
done
clear
D)
\[0.79g\]
done
clear
View Answer play_arrow
question_answer 88) If 300 ml. of a gas at \[{{27}^{o}}C\] is cooled to \[-{{3}^{o}}C\] at constant pressure. The final volume of the gas is:
A)
270 ml
done
clear
B)
630 ml
done
clear
C)
200 ml
done
clear
D)
430 ml
done
clear
View Answer play_arrow
question_answer 89) For equilibrium \[2HI{{H}_{2}}+{{I}_{2}}\]. When \[{{H}_{2}}\] is added at constant temperature, the:
A)
value of \[{{K}_{P}}\] increases
done
clear
B)
value of \[{{K}_{P}}\] decreases
done
clear
C)
degree of dissociation of \[HI\] increases
done
clear
D)
degree of dissociation of \[HI\] decreases
done
clear
View Answer play_arrow
question_answer 90) If the rate constant of a first order reaction is \[{{10}^{-3}}{{\min }^{-1}}.\]The half life period of the reaction is:
A)
46.83 minute
done
clear
B)
642.84 minute
done
clear
C)
69.3 minute
done
clear
D)
693 minute
done
clear
View Answer play_arrow
question_answer 91) If the equilibrium constant for the reaction \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\], then \[{{K}_{C}}\] the equilibrium constant for the reaction \[N{{H}_{3}}\frac{1}{2}{{N}_{2}}+\frac{3}{2}{{H}_{2}}\] is:
A)
\[\frac{1}{K_{C}^{2}}\]
done
clear
B)
\[\sqrt{{{K}_{C}}}\]
done
clear
C)
\[\frac{1}{{{K}_{C}}}\]
done
clear
D)
\[\frac{\sqrt{1}}{{{K}_{C}}}\]
done
clear
View Answer play_arrow
question_answer 92) Which one of the following equation is correct?
A)
\[\Delta G=\Delta H-\Delta T\]
done
clear
B)
\[\Delta G=\Delta H-T\Delta S\]
done
clear
C)
\[\Delta G=\Delta H+T\Delta S\]
done
clear
D)
\[\Delta G=T\Delta S-\Delta H\]
done
clear
View Answer play_arrow
question_answer 93) Time required to deposit 100 g of \[Al\] from an electrolyte cell containing \[A{{l}_{2}}{{O}_{3}}\] by using a current of 125 amperes is:
A)
4820 sec
done
clear
B)
8240.24 sec
done
clear
C)
8577.78 sec
done
clear
D)
6420.44 sec
done
clear
View Answer play_arrow
question_answer 94) The osmotic pressure of a decinormal solution of cane sugar at \[{{0}^{o}}C\] will be : (given R = 0.082 lit atom/k mole)
A)
\[22.4atm\].
done
clear
B)
\[2.24atm\]
done
clear
C)
\[0.224atm\]
done
clear
D)
\[22400atm\]
done
clear
View Answer play_arrow
question_answer 95) Heat of combustion of C, S and \[C{{S}_{2}}\] are \[-393.3,-293.72\]and\[-1108.76\text{ }kJ/mole\]. The heat of formation of \[C{{S}_{2}}\] will be:
A)
\[+128.02\text{ }kJ\]
done
clear
B)
\[-128.02kJ\]
done
clear
C)
\[-212.24\text{ }kJ\]
done
clear
D)
\[+212.24\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 96) The Arrhenius equation representing the effect of temperature on the rate constant of a reaction is:
A)
\[K=In\,\frac{E}{RT}\]
done
clear
B)
\[K=A{{e}^{-E/RT}}\]
done
clear
C)
\[K=A{{e}^{E/RT}}\]
done
clear
D)
\[K={{e}^{-EA/RT}}\]
done
clear
View Answer play_arrow
question_answer 97) pH of \[{{10}^{-4}}M\,\,HCl\] solution is:
A)
2
done
clear
B)
6
done
clear
C)
4
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 98) For the reaction \[Fe{{O}_{(s)}}+C{{O}_{(g)}}F{{e}_{(s)}}+C{{O}_{2}}_{(g)},\,\,{{K}_{C}}=5.0.\] If the concentration of CO at equilibrium is \[2.5\times {{10}^{-2}}mol/litre\] at \[{{100}^{o}}C\]. Then the equilibrium concentration of \[C{{O}_{2}}\] will be:
A)
\[1.25\times {{10}^{-4}}m/L\]
done
clear
B)
\[12.5\times {{10}^{-2}}m/L\]
done
clear
C)
\[2.50\times {{10}^{-3}}m/L\]
done
clear
D)
\[2.50\times {{10}^{-4}}m/L\]
done
clear
View Answer play_arrow
question_answer 99) The volume of \[\frac{M}{10}NaOH\] required to neutralise \[20mL\] of \[\frac{M}{10}NaOH\],, solution is:
A)
\[40\text{ }mL\]
done
clear
B)
\[20\text{ }mL\]
done
clear
C)
\[80\text{ }mL\]
done
clear
D)
\[10\text{ }mL\]
done
clear
View Answer play_arrow
question_answer 100) The vapour pressure of benzene at a certain temperature is 640 mm of Hg. If 2.175 g of a non-volatile solid is added to 39.08 g of benzene, the vapour pressure of solution is 600 mm of Hg. The molecular weight of the solid substance will be:
A)
\[48.60\]
done
clear
B)
\[44.2\]
done
clear
C)
\[64.26\]
done
clear
D)
\[69.45\]
done
clear
View Answer play_arrow
question_answer 101) The 1992 Nobel prize for medicine was awarded to H. Fischer and Edwin J. Krebs for their :
A)
human genome project
done
clear
B)
drug designing involving inhibition of DNA synthesis of the pathogen
done
clear
C)
reversible protein phosphorylation as a biological regulation mechanism
done
clear
D)
isolation of the gene for a human disease
done
clear
View Answer play_arrow
question_answer 102) When yeast ferments glucose, the products obtained are?
A)
Ethanol and water
done
clear
B)
Water and \[C{{O}_{2}}\]
done
clear
C)
Ethanol and\[C{{O}_{2}}\]
done
clear
D)
Methanol and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 103) Mycorrhiza exhibits the phenomenon of:
A)
antagonism
done
clear
B)
endemism
done
clear
C)
parasitism
done
clear
D)
symbiosis
done
clear
View Answer play_arrow
question_answer 104) Initiation codon in eukaryotes is :
A)
AUG
done
clear
B)
UAG
done
clear
C)
GAU
done
clear
D)
AGU
done
clear
View Answer play_arrow
question_answer 105) The first phase in the breakdown of glucose in animal cell is:
A)
glycolysis
done
clear
B)
ETS
done
clear
C)
fermentation
done
clear
D)
Krebs cycle
done
clear
View Answer play_arrow
question_answer 106) Haploid plants can be obtained by culturing:
A)
young leaves
done
clear
B)
endosperm
done
clear
C)
pollen grains
done
clear
D)
root tips
done
clear
View Answer play_arrow
question_answer 107) Hypanthodium is a specialized type of:
A)
thalamus
done
clear
B)
ovary
done
clear
C)
fruit
done
clear
D)
inflorescence
done
clear
View Answer play_arrow
question_answer 108) Colour blindness results from :
A)
absence of rods
done
clear
B)
absence of cones
done
clear
C)
absence of eyelids
done
clear
D)
inverted retina
done
clear
View Answer play_arrow
question_answer 109) The cells of bacterium Staphylococcus remain arranged in the form of:
A)
plate
done
clear
B)
cube
done
clear
C)
irregular
done
clear
D)
chain
done
clear
View Answer play_arrow
question_answer 110) Who credited with the introduction of binomial system of nomenclature of plants?
A)
Linnaeus
done
clear
B)
John Ray
done
clear
C)
Bentham and Hooker
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 111) In embryonic stage RBCs develop in ;
A)
liver and kidney
done
clear
B)
liver and spleen
done
clear
C)
spleen and kidney
done
clear
D)
liver and pancreas
done
clear
View Answer play_arrow
question_answer 112) Eutherian nammals are :
A)
oviparous
done
clear
B)
viviparous
done
clear
C)
ovoviviparous
done
clear
D)
both a and c
done
clear
View Answer play_arrow
question_answer 113) Guttation occurs through :
A)
stomata
done
clear
B)
hydathodes
done
clear
C)
root hairs
done
clear
D)
flower buds
done
clear
View Answer play_arrow
question_answer 114) Cells of liver which .act as phagocytes are?
A)
Deiter cells
done
clear
B)
Kupffer cells
done
clear
C)
Hensen cells
done
clear
D)
Aciner cells
done
clear
View Answer play_arrow
question_answer 115) Lac is produced by :
A)
faeces of lac insect
done
clear
B)
secretion from body
done
clear
C)
excretion from body
done
clear
D)
excess food oozing out of body
done
clear
View Answer play_arrow
question_answer 116) Which one of the following plant hormones is known as a stress hormone?
A)
Gibberellins
done
clear
B)
Kinetin
done
clear
C)
Auxin
done
clear
D)
Abscisic acid
done
clear
View Answer play_arrow
question_answer 117) Which one of the following statements is true for the enzymes ?
A)
All enzymes are protein
done
clear
B)
All proticns are enzymes
done
clear
C)
All enzymes are not protein
done
clear
D)
All unzymes are vitamins
done
clear
View Answer play_arrow
question_answer 118) Sexual reproduction in Spirogyra is morphologically characterized by:
A)
oogamy
done
clear
B)
anisogamy
done
clear
C)
isogamy
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 119) 0.1 M solution of a solute has a water potential of:
A)
- 2.3 bars
done
clear
B)
0 bar
done
clear
C)
22.4 bars
done
clear
D)
+ 2.3 bars
done
clear
View Answer play_arrow
question_answer 120) Kala-azar is transmitted by :
A)
tse-tse fly
done
clear
B)
dragon fly
done
clear
C)
sand fly
done
clear
D)
fruit fly
done
clear
View Answer play_arrow
question_answer 121) Chromosome theory of sex determination was proposed by:
A)
Bridges
done
clear
B)
Balbiani
done
clear
C)
Gold Schmidt
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 122) Biological magnification refers to:
A)
concentration of insecticide in animals
done
clear
B)
concentration of organ ophosphate in plants
done
clear
C)
photography in laboratory
done
clear
D)
increase in number of animals and plants in ecosystem
done
clear
View Answer play_arrow
question_answer 123) Peridem is made up of:
A)
phellem
done
clear
B)
phellogen
done
clear
C)
phelloderm
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 124) A diseased man marries a normal woman and they get three daughters and five sons. All the daughters were diseased and sons were normal. The gene of this disease is :
A)
sex-linked dominant
done
clear
B)
Sex-linked recessive
done
clear
C)
Sex-limited characters
done
clear
D)
Autosomal dominant
done
clear
View Answer play_arrow
question_answer 125) Monadelphous condition of stamens is found in:
A)
Malvaceae
done
clear
B)
Cyperaceae
done
clear
C)
Cruciferae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 126) In which animal nerve cell is present but brain is absent:
A)
Sponge
done
clear
B)
Cockroach
done
clear
C)
Earthworm
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 127) Which of the following is a correct match?
A)
Downs syndrome-21st chromosome
done
clear
B)
Sickle cell anaemia-X-chromosome
done
clear
C)
Haemophilia-y-chromomosome
done
clear
D)
Parkinson disease-X and Y chromosome
done
clear
View Answer play_arrow
question_answer 128) Mainly which type of hormones control the menstrual cycle in human beings?
A)
FSH
done
clear
B)
LH
done
clear
C)
FSH, LH and Estrogen
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 129) Which of the following is used in thyroid cancer?
A)
\[{{I}^{131}}\]
done
clear
B)
\[R{{a}^{224}}\]
done
clear
C)
\[{{U}^{238}}\]
done
clear
D)
\[{{C}^{14}}\]
done
clear
View Answer play_arrow
question_answer 130) Continuous bleeding from an injured part of body due to deficiency of:
A)
Vit. A
done
clear
B)
Vit. B
done
clear
C)
Vit. K
done
clear
D)
Vit. E
done
clear
View Answer play_arrow
question_answer 131) ATP is:
A)
nucleotide
done
clear
B)
nucleoside
done
clear
C)
nucleic acid
done
clear
D)
hormone
done
clear
View Answer play_arrow
question_answer 132) RR-21 is a variety of:
A)
Wheat
done
clear
B)
Gram
done
clear
C)
Rice
done
clear
D)
Sugarcane
done
clear
View Answer play_arrow
question_answer 133) Book lungs are respiratory organs of:
A)
Scorpion
done
clear
B)
Birds
done
clear
C)
Fishes
done
clear
D)
Earthworm
done
clear
View Answer play_arrow
question_answer 134) The cranial capacity of Java ape man is :
A)
1700 cc
done
clear
B)
1450 cc
done
clear
C)
450 cc
done
clear
D)
900 cc
done
clear
View Answer play_arrow
question_answer 135) Pace-maker is:
A)
SA-node
done
clear
B)
AV-node
done
clear
C)
SV-node
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 136) Heterothellism was discovered in :
A)
Mucor
done
clear
B)
Aspergillus
done
clear
C)
Agarcuc
done
clear
D)
Albugo
done
clear
View Answer play_arrow
question_answer 137) Parasitic alga is :
A)
Chara
done
clear
B)
Oedogonium
done
clear
C)
Anabaena
done
clear
D)
Cephaleuros
done
clear
View Answer play_arrow
question_answer 138) TMV has genetic material as:
A)
ss RNA
done
clear
B)
ds RNA
done
clear
C)
ss DNA
done
clear
D)
ds DNA
done
clear
View Answer play_arrow
question_answer 139) Golden age of reptiles is:
A)
Coenozoic era
done
clear
B)
Palaeozoic era
done
clear
C)
Mesozoic era
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 140) Number of wild life is continuously decreasing. What is the main reason of this?
A)
Predation
done
clear
B)
Cutting down of forests
done
clear
C)
Destruction of habitats
done
clear
D)
Hunting
done
clear
View Answer play_arrow
question_answer 141) Seed dormancy is due to :
A)
ethylene
done
clear
B)
abscisic acid
done
clear
C)
IAA
done
clear
D)
Starch
done
clear
View Answer play_arrow
question_answer 142) Ediple part in Mango is :
A)
mesoc.irp
done
clear
B)
epicarp
done
clear
C)
cndocarp
done
clear
D)
epidermis
done
clear
View Answer play_arrow
question_answer 143) Maximum green house gases is released by which country ?
A)
India
done
clear
B)
USA
done
clear
C)
England
done
clear
D)
Germany
done
clear
View Answer play_arrow
question_answer 144) Which of the following are homologus organs?
A)
Wings of Birds and Locust
done
clear
B)
Wings of Bat and Butterfly
done
clear
C)
Leg of Cockroach and Frog
done
clear
D)
Wings of Birds and pectoral fins of Fish
done
clear
View Answer play_arrow
question_answer 145) Change in the sequence of nucleotide in DNA is called :
A)
mutagen
done
clear
B)
mutation
done
clear
C)
recombination
done
clear
D)
translation
done
clear
View Answer play_arrow
question_answer 146) A food chain starts with :
A)
nitrogen fixing organisms
done
clear
B)
photosynthesising organisms
done
clear
C)
respiration
done
clear
D)
decomposers
done
clear
View Answer play_arrow
question_answer 147) Nepenthes is a :
A)
primary producer
done
clear
B)
consumer
done
clear
C)
both a and b
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 148) Dudhawa National park is located in :
A)
Madhya Pradesh
done
clear
B)
Himachal Pradesh
done
clear
C)
Arunachal Pradesh
done
clear
D)
Uttar Pradesh
done
clear
View Answer play_arrow
question_answer 149) Glissons capsule is found in :
A)
pancreas
done
clear
B)
liver
done
clear
C)
gall bladder
done
clear
D)
heart
done
clear
View Answer play_arrow
question_answer 150) Which of the following is sexually transmitted disease (STD) ?
A)
Malaria
done
clear
B)
Syphilis
done
clear
C)
Goitre
done
clear
D)
TB
done
clear
View Answer play_arrow
question_answer 151) A ribosome is composed of :
A)
DNA, RNA and protein
done
clear
B)
only protein
done
clear
C)
protein and RNA
done
clear
D)
RNA and DNA
done
clear
View Answer play_arrow
question_answer 152) A nucleoside differ from a nucleotide on not having:
A)
phosphate
done
clear
B)
calcium
done
clear
C)
nitrogen base
done
clear
D)
phosphate and sugar
done
clear
View Answer play_arrow
question_answer 153) DNA is present in:
A)
Nucleus only
done
clear
B)
Mitochondria only
done
clear
C)
Chloropbst only
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 154) AIDS can be transmitted by :
A)
blood circulation
done
clear
B)
handshake
done
clear
C)
court ship
done
clear
D)
by using the utensils of infected person
done
clear
View Answer play_arrow
question_answer 155) The chief difference between RBCs of Human and Frog :
A)
only Human RBCs have haemoglobin
done
clear
B)
Human RBCs have more nuclei
done
clear
C)
Human RBCs are without nucleus
done
clear
D)
Frog RBCs are without nucleus
done
clear
View Answer play_arrow
question_answer 156) The cell theory was proposed by :
A)
Schleiden and Schwann
done
clear
B)
Hugo dc Vries
done
clear
C)
Robert Hooke
done
clear
D)
R. Virchow
done
clear
View Answer play_arrow
question_answer 157) Which of the plant yield sunhemp ?
A)
Cnniinbis
done
clear
B)
Crotaleria
done
clear
C)
Corchorus
done
clear
D)
Hibiscus
done
clear
View Answer play_arrow
question_answer 158) LDS is obtained from :
A)
Raiiwoifin
done
clear
B)
Cinchofvi
done
clear
C)
Cannabis
done
clear
D)
daviceps
done
clear
View Answer play_arrow
question_answer 159) Which group of the following scientists discovered the EMP pathway of glycolysis :
A)
Embdcn, Meyrrliof and Parnas
done
clear
B)
Emerson, Hoffman and Peterson
done
clear
C)
Embden, Morrison and Pitcher
done
clear
D)
Avery, Mcleon and McCarthy
done
clear
View Answer play_arrow
question_answer 160) Rennin is used in cheese industry is :
A)
antibiotic
done
clear
B)
alkaloid
done
clear
C)
enzyme
done
clear
D)
inhibitor
done
clear
View Answer play_arrow
question_answer 161) Plasmodium in man is inoculated by
A)
Anopheles male
done
clear
B)
Anopheles female
done
clear
C)
Culex male
done
clear
D)
Ades female
done
clear
View Answer play_arrow
question_answer 162) Bordeaux mixture is used as a:
A)
fertilizer
done
clear
B)
fungicide
done
clear
C)
rodenticide
done
clear
D)
soil testing chemical
done
clear
View Answer play_arrow
question_answer 163) Blood of which blood group can be given to a person with blood group A ?
A)
A only
done
clear
B)
O only
done
clear
C)
A or O
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 164) Which one of the following bacterium has potential for nitrogen fixation?
A)
Nitrosomonas
done
clear
B)
Azolla
done
clear
C)
Nitrobacter
done
clear
D)
Rhizobium
done
clear
View Answer play_arrow
question_answer 165) In meiotic division, centromere divide during:
A)
diplotene
done
clear
B)
metaphase-I
done
clear
C)
pachytene
done
clear
D)
anaphase-II
done
clear
View Answer play_arrow
question_answer 166) The first \[C{{O}_{2}}\]acceptor in \[{{C}_{4}}\]plants is:
A)
PEP
done
clear
B)
RuDP
done
clear
C)
PGA
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 167) Calvin cycle occurs in :
A)
chloroplnst
done
clear
B)
cytoplasm
done
clear
C)
mitochondria
done
clear
D)
glyoxysome
done
clear
View Answer play_arrow
question_answer 168) Which one of the following hormones is not found in plants?
A)
2, 4-D
done
clear
B)
\[G{{A}_{2}}\]
done
clear
C)
Gibberellin
done
clear
D)
IAA
done
clear
View Answer play_arrow
question_answer 169) A gene is made up of:
A)
DNA
done
clear
B)
RNA
done
clear
C)
either RNA or DNA
done
clear
D)
amino acids Generally gene is made up of DNA but in some viruses gene is made up of RNA.
done
clear
View Answer play_arrow
question_answer 170) Chlorophyll is soluble in:
A)
water
done
clear
B)
organic solvents
done
clear
C)
both a and b
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 171) A contraceptive pill contains ;
A)
progesterone and estrogen
done
clear
B)
spermicidal salts
done
clear
C)
chemicals that cause automatic abortion
done
clear
D)
chemicals that prevents fertilization of ovum
done
clear
View Answer play_arrow
question_answer 172) Jully 11 is :
A)
World Environment Day
done
clear
B)
World population Day
done
clear
C)
World AIDS Day
done
clear
D)
World Education Day
done
clear
View Answer play_arrow
question_answer 173) B.C.G. vaccine works against:
A)
Bacillus Comma, Gram
done
clear
B)
Diphtheria, Pertussis and Tetanus
done
clear
C)
Measles, Mumps and Rubella
done
clear
D)
T.B. Bacillus Calmette-Guerin
done
clear
View Answer play_arrow
question_answer 174) Sweet potato is the modification of:
A)
tap root
done
clear
B)
stem
done
clear
C)
leaf
done
clear
D)
adventitious root
done
clear
View Answer play_arrow
question_answer 175) Amino acid binding site in f-RNA is:
A)
5 end
done
clear
B)
anticodon loop
done
clear
C)
CCA 3 end
done
clear
D)
DHU loop
done
clear
View Answer play_arrow
question_answer 176) Tissue present in an annual ring is :
A)
secondary xylem and phloem
done
clear
B)
primary xylem and phloem
done
clear
C)
secondary xylem only
done
clear
D)
primary phloem and secondary xylem
done
clear
View Answer play_arrow
question_answer 177) The termination codons are:
A)
UAU, UAC, UCU
done
clear
B)
UCU, UGC, UGG
done
clear
C)
UAA, UAG, UGA
done
clear
D)
UCC, UCA, UCG
done
clear
View Answer play_arrow
question_answer 178) Which of the following is not an insect?
A)
Mosquito
done
clear
B)
Ant
done
clear
C)
Spider
done
clear
D)
Termite
done
clear
View Answer play_arrow
question_answer 179) Which of the following is not a flower?
A)
Rose
done
clear
B)
Lotus
done
clear
C)
Sunflower
done
clear
D)
Passion flower
done
clear
View Answer play_arrow
question_answer 180) A plasmolysed cell can be deplasmolysed by placing it in :
A)
isotonic solution
done
clear
B)
hypertonic solution
done
clear
C)
saturated solution
done
clear
D)
pure water or hypotonic solution
done
clear
View Answer play_arrow
question_answer 181) In human body urea is produced as an excretory product in the :
A)
liver
done
clear
B)
kidneys
done
clear
C)
urinary bladder
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 182) Electron microscope is invented by :
A)
Knoll and Ruska
done
clear
B)
Robert Hooke
done
clear
C)
Leeuwonhoek
done
clear
D)
Pasteur
done
clear
View Answer play_arrow
question_answer 183) HPLC stands for :
A)
high performance liquid chromatography
done
clear
B)
heavy partical liquid chromatography
done
clear
C)
heat power liquid chromatography
done
clear
D)
high potential low chromatography
done
clear
View Answer play_arrow
question_answer 184) Cryobiology deals with:
A)
temperature effect
done
clear
B)
physiology
done
clear
C)
anatomy
done
clear
D)
characteristics of biomolecule
done
clear
View Answer play_arrow
question_answer 185) Penicillin an antibiotic was discovered by:
A)
Pasteur
done
clear
B)
Koch
done
clear
C)
Flemming
done
clear
D)
Kurosawa
done
clear
View Answer play_arrow
question_answer 186) In human beings, the number of cranial nerves are:
A)
10 pairs
done
clear
B)
12 pairs
done
clear
C)
6 pairs
done
clear
D)
20 pairs
done
clear
View Answer play_arrow
question_answer 187) Blood pressure of healthy person is :
A)
120/80
done
clear
B)
140/60
done
clear
C)
90/60
done
clear
D)
200/130
done
clear
View Answer play_arrow
question_answer 188) Agar-agar which is used in tissue culture is obtained from a :
A)
fungi
done
clear
B)
algae
done
clear
C)
pteridophyte
done
clear
D)
bryophyte
done
clear
View Answer play_arrow
question_answer 189) Molecular scissors used in genetic engineering is:
A)
DNA polymerase
done
clear
B)
DNA ligase
done
clear
C)
restriction endonuclease
done
clear
D)
helicase
done
clear
View Answer play_arrow
question_answer 190) The species of animal protected in Kaziranga sanctuary is:
A)
Panthera Sea
done
clear
B)
Rhinoceros uniforms
done
clear
C)
Macaco mulatto
done
clear
D)
Panthera tigris
done
clear
View Answer play_arrow
question_answer 191) The largest gland is the human body is:
A)
pancreas
done
clear
B)
brain
done
clear
C)
gall bladder
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 192) Haversian systems are found in the bones of:
A)
Pigeon
done
clear
B)
Panther
done
clear
C)
Pipe fish
done
clear
D)
Python
done
clear
View Answer play_arrow
question_answer 193) R.Q of sprouting potato tubers will be :
A)
1
done
clear
B)
< 1
done
clear
C)
> 1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 194) Fem prothallus develops from:
A)
spore
done
clear
B)
oospore
done
clear
C)
elaters
done
clear
D)
antherozoid
done
clear
View Answer play_arrow
question_answer 195) Winged pollen grain is the characteristic feature of:
A)
Cycas
done
clear
B)
Ephedra
done
clear
C)
Gnetum
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 196) In which bee wax glands are found?
A)
Drone
done
clear
B)
Workers
done
clear
C)
Queen bee
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 197) Diabetes mellitus is due to lack of:
A)
starch in blood
done
clear
B)
trypsin in pancreatic Juice
done
clear
C)
ADH reaching in kidneys
done
clear
D)
insulin in blood
done
clear
View Answer play_arrow
question_answer 198) Deficiency of vit. C causes:
A)
pellagra
done
clear
B)
diabetes
done
clear
C)
haemophilia
done
clear
D)
scurvy
done
clear
View Answer play_arrow
question_answer 199) What is introduced in polio vaccine ?
A)
Antibodies
done
clear
B)
Antigen
done
clear
C)
Antibiotics
done
clear
D)
Bacteriostatic agent
done
clear
View Answer play_arrow
question_answer 200) Chromosomes are lined up in the equatorial plane during?
A)
metaphase
done
clear
B)
annphase
done
clear
C)
telophase
done
clear
D)
interphase
done
clear
View Answer play_arrow
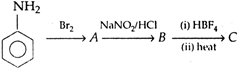 Identify C.
Identify C.
