-
question_answer1) If the cold junction of a thermo-couple is kept at \[{{0}^{\text{o}}}C\] and the hot junction is kept at \[{{T}^{\text{o}}}C,\] then the relation between neutral temperature \[({{T}_{n}})\] and temperature of inversion \[({{T}_{i}})\] is:
A)
\[{{T}_{n}}=\frac{{{T}_{i}}}{2}\]
done
clear
B)
\[{{T}_{n}}=2{{T}_{i}}\]
done
clear
C)
\[{{T}_{n}}={{T}_{i}}-T\]
done
clear
D)
\[{{T}_{n}}={{T}_{i}}+T\]
done
clear
View Answer play_arrow
-
question_answer2) Nickel shows ferromagnetic property at room temperature. If the temperature is increased beyond Curie temperature, then it will show:
A)
paramagnetism
done
clear
B)
anti-ferromagnetism
done
clear
C)
no magnetic property
done
clear
D)
diamagnetism
done
clear
View Answer play_arrow
-
question_answer3) In radioactive decay process, the negatively charged emitted \[\beta \]-particles are:
A)
the electrons present inside the nucleus
done
clear
B)
the electrons produced as a result of the decay of neutrons inside the nucleus
done
clear
C)
the electrons produced as a result of collisions between atoms
done
clear
D)
the electrons orbiting around the nucleus
done
clear
View Answer play_arrow
-
question_answer4) A small coin is resting on the bottom of a beaker filled with a liquid. A ray of light from the coin travels upto the surface of the liquid and moves along its surface (see figure).
A)
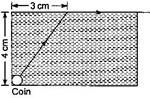
How fast is the light travelling in the liquid? \[1.8\times {{10}^{8}}\,m/s\]
done
clear
B)
\[2.4\times {{10}^{8}}\]
done
clear
C)
\[3.0\times {{10}^{8}}\,m/s\]
done
clear
D)
\[1.2\times {{10}^{8}}\,m/s\]
done
clear
View Answer play_arrow
-
question_answer5) What is the value of inductance I for which the current is a maximum in a series LCR circuit with C = 10 \[\mu \]F------------------------ and to \[\omega \] \[=1000\,{{s}^{-1}}\]?
A)
100 mH
done
clear
B)
1 mh
done
clear
C)
Cannot be calculated unless R is known
done
clear
D)
10 mH
done
clear
View Answer play_arrow
-
question_answer6) Three point charges +q, -2q and +q are placed at points (x = 0, y = a, z = 0), (x = 0, y = 0, z = 0) and (x = a, y = 0, z = 0), respectively. The magnitude and direction of the electric dipole moment vector of this charge assembly are:
A)
\[\sqrt{2}\] qa along +y direction
done
clear
B)
\[\sqrt{2}\] qa along the line joining points (x = 0, y = 0, z = 0) and (x = a, y = a, z = 0)
done
clear
C)
qa along the line joining points (x = 0, y = 0, z = 0) and (x = a, y = a, z = 0)
done
clear
D)
\[\sqrt{2}\]qa along +x direction
done
clear
View Answer play_arrow
-
question_answer7) A nucleus \[_{Z}^{A}X\] has mass represented by M(A, Z). If \[{{M}_{p}}\] and \[{{M}_{n}}\] denote the mass of proton and neutron respectively and BE the binding energy (in MeV), then:
A)
\[BE=[M(A,\,Z)-Z{{M}_{p}}-(A-Z){{M}_{n}}]{{c}^{2}}\]
done
clear
B)
\[BE=[Z{{M}_{p}}+(A-Z){{M}_{n}}-M(A,\,Z)]{{c}^{2}}\]
done
clear
C)
\[BE=[Z{{M}_{p}}+A{{M}_{n}}-M(A,\,Z)]{{c}^{2}}\]
done
clear
D)
\[BE=M\,(A,\,Z)-Z{{M}_{p}}\,-(A-Z){{M}_{n}}\]
done
clear
View Answer play_arrow
-
question_answer8) The position x of a particle with respect to time t along x-axis is given by \[x=9{{t}^{2}}-{{t}^{3}}\] where x is in metre and t in second. What will be the position of this particle when it achieves maximum speed along the +x direction?
A)
32 m
done
clear
B)
54 m
done
clear
C)
81 m
done
clear
D)
24 m
done
clear
View Answer play_arrow
-
question_answer9)
The total power dissipated in watts in the circuit shown here 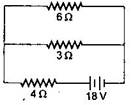
A)
16
done
clear
B)
40
done
clear
C)
54
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer10)
In the following circuit, the output Y for all possible inputs A and B is expressed by the truth table: 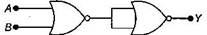
A)
A B Y 0 0 0 0 1 0 1 0 0 1 1 1
done
clear
B)
A B Y 0 0 1 0 0 1 1 0 1 1 1 0
done
clear
C)
A B Y 0 0 1 0 1 0 1 0 0 1 1 0
done
clear
D)
A B Y 0 0 0 0 1 1 1 1 1
done
clear
View Answer play_arrow
-
question_answer11) Assuming the sun to have a spherical outer surface of radius r, radiating like a black body at temperature \[{{t}^{\text{o}}}C,\] the power received by a unit surface, (normal to the incident rays) at a distance R from the centre of the sun is:
A)
\[\frac{4\pi {{r}^{2}}\sigma \,{{t}^{4}}}{{{R}^{2}}}\]
done
clear
B)
\[\frac{{{r}^{2}}\,\sigma \,{{(t+273)}^{4}}}{4\pi {{R}^{2}}}\]
done
clear
C)
\[\frac{16\,{{\pi }^{2}}\,{{r}^{2}}\,\sigma {{t}^{4}}}{{{R}^{2}}}\]
done
clear
D)
\[\frac{{{r}^{2}}\,\sigma \,{{(t+273)}^{4}}}{{{R}^{2}}}\] where \[\sigma \] is the Stefan's constant.
done
clear
View Answer play_arrow
-
question_answer12) A particle starting from the origin (0, 0) moves in a straight line in die (x, y) plane. Its coordinates at a later time are \[(\sqrt{3},\,\,3)\]. The path of the particle makes with the x-axis an angle of:
A)
\[{{30}^{\text{o}}}\]
done
clear
B)
\[{{45}^{\text{o}}}\]
done
clear
C)
\[{{60}^{\text{o}}}\]
done
clear
D)
\[{{0}^{\text{o}}}\]
done
clear
View Answer play_arrow
-
question_answer13) If the nucleus \[_{13}^{27}Al\] has a nuclear radius of about 3.6 fm, then \[_{52}^{125}Te\] would have its radius approximately as:
A)
6.0 fm
done
clear
B)
9.6 fm
done
clear
C)
12.0 fm
done
clear
D)
4.8 fm
done
clear
View Answer play_arrow
-
question_answer14) A wheel has angular acceleration of \[3.0\,\,rad/{{s}^{2}}\] and an initial angular speed of 2.00 rad/s. In a time of 2 s it has rotated through an angle (in radian) of:
A)
6
done
clear
B)
10
done
clear
C)
12
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer15) The resistance of an ammeter is \[13\,\,\Omega \] and its scale is graduated for a current upto 100 A. After an additional shunt has been connected to this ammeter it becomes possible to measure currents upto 750 A by this meter. The value of shunt resistance is:
A)
\[20\,\,\Omega \]
done
clear
B)
\[2\,\,\Omega \]
done
clear
C)
\[0.2\,\,\Omega \]
done
clear
D)
\[2\,k\,\,\Omega \]
done
clear
View Answer play_arrow
-
question_answer16) Under the influence of a uniform magnetic field a charged particle is moving in a circle of radius R with constant speed v. The time period of the motion:
A)
depends on v and not on R
done
clear
B)
depends on both R and v
done
clear
C)
is independent of both R and v
done
clear
D)
depends on R and not on v
done
clear
View Answer play_arrow
-
question_answer17) The primary and secondary coils of a transformer have 50 and 1500 turns respectively. If the magnetic flux \[\phi \] linked with the primary coil is given by \[\phi ={{\phi }_{0}}+4t\], where \[\phi \] is in weber, t is time in second and \[{{\phi }_{0}}\] is a constant, the output voltage across the secondary coil is:
A)
90 V
done
clear
B)
120 V
done
clear
C)
220 V
done
clear
D)
30 V
done
clear
View Answer play_arrow
-
question_answer18)
Two condensers, one of capacity C and the other of capacity \[\frac{C}{2}\], are connected to a V volt battery, as shown. 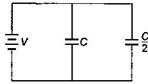 The work done in charging fully both the condensers is:
The work done in charging fully both the condensers is:
A)
\[2C{{V}^{2}}\]
done
clear
B)
\[\frac{1}{4}\,C{{V}^{2}}\]
done
clear
C)
\[\frac{3}{4}\,C{{V}^{2}}\]
done
clear
D)
\[\frac{1}{2}\,C{{V}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer19)
A uniform rod AB of length \[l\] and mass m is free to rotate about point A. The rod is released from rest in the horizontal position. Given that the moment of inertia of the rod about A is \[\frac{m{{l}^{2}}}{3}\], the initial angular acceleration of the rod will be: 
A)
\[\frac{2g}{3l}\]
done
clear
B)
\[mg\frac{l}{2}\]
done
clear
C)
\[\frac{3}{2}gl\]
done
clear
D)
\[\frac{3g}{2l}\]
done
clear
View Answer play_arrow
-
question_answer20) The frequency of a light wave in a material is \[2\times {{10}^{14}}Hz\] and wavelength is \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]. The refractive index of material will be:
A)
1.40
done
clear
B)
1.50
done
clear
C)
3.00
done
clear
D)
1.33
done
clear
View Answer play_arrow
-
question_answer21)
In the energy band diagram of a material shown below, the open circles and filled circles denote holes and electrons respectively. The material is a/an: 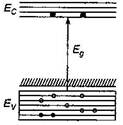
A)
p-type semiconductor
done
clear
B)
insulator
done
clear
C)
metal
done
clear
D)
n-type semiconductor
done
clear
View Answer play_arrow
-
question_answer22) A car moves from-X to Y with a uniform speed \[{{v}_{u}}\] and returns to Y with a uniform speed \[{{v}_{d}}\]. The average speed for this round trip is:
A)
\[\frac{2\,{{v}_{d}}\,{{v}_{u}}}{{{v}_{d}}+{{v}_{u}}}\]
done
clear
B)
\[\sqrt{{{v}_{u}}\,{{v}_{d}}}\]
done
clear
C)
\[\frac{{{v}_{d}}\,{{v}_{u}}}{{{v}_{d}}+{{v}_{u}}}\]
done
clear
D)
\[\frac{{{v}_{u}}+{{v}_{d}}}{2}\]
done
clear
View Answer play_arrow
-
question_answer23) A particle executes simple harmonic oscillation with an amplitude a. The period of oscillation is T. The minimum time taken by the particle to travel half of the amplitude from the equilibrium position is:
A)
\[\frac{T}{4}\]
done
clear
B)
\[\frac{T}{8}\]
done
clear
C)
\[\frac{T}{12}\]
done
clear
D)
\[\frac{T}{2}\]
done
clear
View Answer play_arrow
-
question_answer24) A 5 W source emits monochromatic light of wavelength \[5000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. When the source is moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of:
A)
4
done
clear
B)
8
done
clear
C)
16
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer25)
A block B is pushed momentarily along a horizontal surface with an initial velocity v. If \[\mu \] is the coefficient of sliding friction between B and the surface, blocks will come to rest after a time: 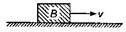
A)
\[\frac{v}{g\mu }\]
done
clear
B)
\[\frac{g\mu }{v}\]
done
clear
C)
\[\frac{g}{v}\]
done
clear
D)
\[\frac{v}{g}\]
done
clear
View Answer play_arrow
-
question_answer26) Two radioactive substances A and B have decay constants 5\[\lambda \], and \[\lambda \] respectively. At t = 0 they have the same number of nuclei. The ratio of number of nuclei of A to those of B will be \[{{\left( \frac{1}{e} \right)}^{2}}\] after a time interval:
A)
\[\frac{1}{4\lambda }\]
done
clear
B)
4\[\lambda \]
done
clear
C)
2\[\lambda \]
done
clear
D)
\[\frac{1}{2\lambda }\]
done
clear
View Answer play_arrow
-
question_answer27)
A hollow cylinder has a charge q coulomb within it. If \[\phi \] is the electric flux in unit of voltmeter associated with the curved surface B, the flux linked with the plane surface A in unit of voltmeter will be: 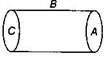
A)
\[\frac{1}{2}\left( \frac{q}{{{\varepsilon }_{0}}}-\phi \right)\]
done
clear
B)
\[\frac{q}{2\,{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{\phi }{3}\]
done
clear
D)
\[\frac{q}{{{\varepsilon }_{0}}}-\phi \]
done
clear
View Answer play_arrow
-
question_answer28) A transformer is used to light a 100 W and 110 V lamp from a 220 V mains. If the main current is 0.5 A, the efficiency of the transformer is approximately:
A)
30%
done
clear
B)
50%
done
clear
C)
90%
done
clear
D)
10%
done
clear
View Answer play_arrow
-
question_answer29) A charged particle (charge q) is moving in a circle of radius R with uniform speed v. The associated magnetic moment u is given by:
A)
\[\frac{qvR}{2}\]
done
clear
B)
\[qv{{R}^{2}}\]
done
clear
C)
\[\frac{qv{{R}^{2}}}{2}\]
done
clear
D)
\[qvR\]
done
clear
View Answer play_arrow
-
question_answer30) A particle of mass m moves in the XY plane with a velocity v along the straight line AB. if the angular momentum of the particle with respect to origin O is \[{{L}_{A}}\] when it is at A and \[{{L}_{B}}\] when it is at B, then:
A)
\[{{L}_{A}}>{{L}_{B}}\]
done
clear
B)
\[{{L}_{A}}={{L}_{B}}\]
done
clear
C)
the relationship between \[{{L}_{A}}\] and \[{{L}_{B}}\] depends upon the slope of the line AB
done
clear
D)
\[{{L}_{A}}<{{L}_{B}}\]
done
clear
View Answer play_arrow
-
question_answer31) The total energy of electron in the ground state of hydrogen atom is -13.6 eV. The kinetic energy of an electron in the first excited state is:
A)
3.4 eV
done
clear
B)
6.8 eV
done
clear
C)
13.6 eV
done
clear
D)
1.7 eV
done
clear
View Answer play_arrow
-
question_answer32) A steady current of 1.5 A flows through a copper voltameter for 10 min. If the electrochemical equivalent of copper is \[30\times {{10}^{-5}}g\,{{C}^{-1}}\] the mass of copper deposited on the electrode will be:
A)
0.40 g
done
clear
B)
0.50 g
done
clear
C)
0.67 g
done
clear
D)
0.27 g
done
clear
View Answer play_arrow
-
question_answer33) In a mass spectrometer used for measuring the masses of ions, the ions are initially accelerated by an electric potential V and then made to describe semicircular paths of radius R using a magnetic field B. If V and B are kept constant, the ratio \[\left( \frac{ch\arg e\,on\,the\,ion}{mass\,of\,the\,ion} \right)\]will be proportional to:
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{{{R}^{2}}}\]
done
clear
C)
\[{{R}^{2}}\]
done
clear
D)
R
done
clear
View Answer play_arrow
-
question_answer34) Three resistances P, Q, R each of \[2\,\Omega \] and an unknown resistance S form the four arms of a Wheatstone's bridge circuit. When a resistance of \[6\,\,\Omega \] is connected in parallel to S the bridge gets balanced. What is the value of S?
A)
\[2\,\Omega \]
done
clear
B)
\[3\,\Omega \]
done
clear
C)
\[6\,\,\Omega \]
done
clear
D)
\[1\,\,\Omega \]
done
clear
View Answer play_arrow
-
question_answer35) The particle executing simple harmonic motion has a kinetic energy \[{{K}_{0}}{{\cos }^{2}}\omega t\]. The maximum values of the potential energy and the total energy are respectively:
A)
0 and \[2\,{{K}_{0}}\]
done
clear
B)
\[\frac{{{K}_{0}}}{2}\] and \[{{K}_{0}}\]
done
clear
C)
\[{{K}_{0}}\] and \[2{{K}_{0}}\]
done
clear
D)
\[{{K}_{0}}\] and \[{{K}_{0}}\]
done
clear
View Answer play_arrow
-
question_answer36) The electric and magnetic field of an electromagnetic wave are:
A)
in phase and parallel to each other
done
clear
B)
in opposite phase and perpendicular to each other
done
clear
C)
in opposite phase and parallel to each other
done
clear
D)
in phase and perpendicular to each other
done
clear
View Answer play_arrow
-
question_answer37)
A mass of 2.0 kg is put on a flat pan attached to a vertical spring fixed on the ground as shown in the figure. The mass of the spring and the pan is negligible. When pressed slightly and released the mass executes a simple harmonic motion. The spring constant is 200 N/m. What should be the minimum amplitude of the motion, so that the mass gets detached from the pan? (Take \[g=10\,m/{{s}^{2}}\]) 

A)
8.0 cm
done
clear
B)
10.0 cm
done
clear
C)
Any value less than 12.0 cm
done
clear
D)
4.0 cm
done
clear
View Answer play_arrow
-
question_answer38) Two satellites of earth, \[{{S}_{1}}\] and \[{{S}_{2}}\], are moving in the same orbit. The mass of \[{{S}_{1}}\] is four times the mass of \[{{S}_{2}}\]. Which one of the following statements is true?
A)
The time period of \[{{S}_{1}}\] is four times that of \[{{S}_{2}}\]
done
clear
B)
The potential energies of earth and satellite in the two cases are equal
done
clear
C)
\[{{S}_{1}}\] and \[{{S}_{2}}\] are moving with the same speed
done
clear
D)
The kinetic energies of the two satellites are equal
done
clear
View Answer play_arrow
-
question_answer39)
Charges +q and -q are placed at points A and B respectively which are a distance 2 L apart, C is the midpoint between A and S. The work done in moving a charge +Q along the semicircle CRD is: 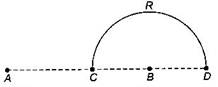
A)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}L}\]
done
clear
B)
\[\frac{qQ}{2\pi {{\varepsilon }_{0}}L}\]
done
clear
C)
\[\frac{qQ}{6\pi {{\varepsilon }_{0}}L}\]
done
clear
D)
\[-\frac{qQ}{6\pi {{\varepsilon }_{0}}L}\]
done
clear
View Answer play_arrow
-
question_answer40) A beam of electrons passes undetected through mutually perpendicular electric and magnetic fields. If the electric field is switched off, and the same magnetic field is maintained, the electrons move:
A)
in an elliptical orbit
done
clear
B)
in a circular orbit
done
clear
C)
along a parabolic path
done
clear
D)
along a straight line
done
clear
View Answer play_arrow
-
question_answer41) A black body is at \[{{727}^{\text{o}}}C\]. It emits energy at a rate which is proportional to:
A)
\[{{(727)}^{2}}\]
done
clear
B)
\[{{(1000)}^{4}}\]
done
clear
C)
\[{{(1000)}^{2}}\]
done
clear
D)
\[{{(727)}^{4}}\]
done
clear
View Answer play_arrow
-
question_answer42) An engine has an efficiency of \[\frac{1}{6}\]. When the temperature of sink is reduced by \[{{62}^{\text{o}}}C,\] its efficiency is doubled. Temperature of the source is:
A)
\[{{124}^{\text{o}}}C\]
done
clear
B)
\[{{37}^{\text{o}}}C\]
done
clear
C)
\[{{62}^{\text{o}}}C\]
done
clear
D)
\[{{99}^{\text{o}}}C\]
done
clear
View Answer play_arrow
-
question_answer43) Monochromatic light of frequency \[6.0\times {{10}^{14}}Hz\] is produced by a laser. The power emitted is \[2\times {{10}^{-3}}W\]. The number of photons emitted, on the average, by the source per second is:
A)
\[5\times {{10}^{15}}\]
done
clear
B)
\[5\times {{10}^{16}}\]
done
clear
C)
\[5\times {{10}^{17}}\]
done
clear
D)
\[5\times {{10}^{14}}\]
done
clear
View Answer play_arrow
-
question_answer44) For a cubic crystal structure which one of the following relations indicating the cell characteristic is correct?
A)
\[a\ne b\ne c\] and \[\alpha \ne \beta \] and \[\gamma \ne {{90}^{o}}\]
done
clear
B)
\[a\ne b\ne c\] and \[\alpha =\beta =\gamma ={{90}^{o}}\]
done
clear
C)
\[a=b=c\] and \[\alpha \ne \beta \ne \gamma ={{90}^{o}}\]
done
clear
D)
\[a=b=c\] and \[\alpha =\beta =\gamma ={{90}^{o}}\]
done
clear
View Answer play_arrow
-
question_answer45) A common emitter amplifier has a voltage gain of 50, an input impedance of \[100\,\Omega \] and an output impedance of\[200\,\Omega \]. The power gain of the amplifier is:
A)
500
done
clear
B)
1000
done
clear
C)
1250
done
clear
D)
100
done
clear
View Answer play_arrow
-
question_answer46) The phase difference between the instantaneous velocity and acceleration of a particle executing simple harmonic motion is:
A)
\[0.5\,\,\pi \]
done
clear
B)
\[\pi \]
done
clear
C)
\[0.707\,\,\pi \]
done
clear
D)
zero
done
clear
View Answer play_arrow
-
question_answer47) A vertical spring with force constant k is fixed on a table. A ball of mass m at a height ft above the free upper end of the spring falls vertically on the spring, so that the spring is compressed by a distance d. The net work done in the process is:
A)
\[mg(h+d)+\frac{1}{2}k{{d}^{2}}\]
done
clear
B)
\[mg(h+d)-\frac{1}{2}k{{d}^{2}}\]
done
clear
C)
\[mg(h-d)-\frac{1}{2}k{{d}^{2}}\]
done
clear
D)
\[mg(h-d)+\frac{1}{2}k{{d}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer48) \[\vec{A}\] and \[\vec{B}\] are two vectors and \[\theta \] is the angle between them, if \[|\vec{A}\times \vec{B}|=\sqrt{3}\,(\vec{A}\,\centerdot \,\mathbf{\vec{B}})\]the value of \[\theta \] is:
A)
\[{{60}^{\text{o}}}\]
done
clear
B)
\[{{45}^{\text{o}}}\]
done
clear
C)
\[{{30}^{\text{o}}}\]
done
clear
D)
\[{{90}^{\text{o}}}\]
done
clear
View Answer play_arrow
-
question_answer49) Dimensions of resistance in an electrical circuit, in terms of dimension of mass M, of length L, of time T and of current I, would be:
A)
\[[M{{L}^{2}}{{T}^{-3}}{{I}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-1}}{{I}^{-1}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-3}}{{I}^{-2}}]\]
done
clear
View Answer play_arrow
-
question_answer50) A particle moving along \[x\]-axis has acceleration \[f\], at time t, given by \[f={{f}_{0}}\left( 1-\frac{t}{T} \right)\], where \[{{f}_{0}}\] and T are constants. The particle at t = 0 has zero velocity. In the time interval between t = 0 and the instant when \[f=0\], the particle's velocity \[({{v}_{x}})\] is:
A)
\[{{f}_{0}}T\]
done
clear
B)
\[\frac{1}{2}{{f}_{0}}{{T}^{2}}\]
done
clear
C)
\[{{f}_{0}}{{T}^{2}}\]
done
clear
D)
\[\frac{1}{2}{{f}_{0}}T\]
done
clear
View Answer play_arrow
-
question_answer51) Calculate the \[pOH\] of a solution at \[{{25}^{\text{o}}}C\] that contains \[1\times {{10}^{-10}}M\] of hydronium ions.
A)
7.00
done
clear
B)
4.00
done
clear
C)
9.00
done
clear
D)
1.00
done
clear
View Answer play_arrow
-
question_answer52) Which of the following will give a pair of enantiomorphs?
A)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]N{{O}_{2}}\]
done
clear
B)
\[[Cr{{(N{{H}_{3}})}_{6}}]\,\,[Co{{(CN)}_{6}}]\]
done
clear
C)
\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
done
clear
D)
\[[Pt{{(N{{H}_{3}})}_{4}}]\,\,[PtC{{l}_{6}}]\] \[(en=N{{H}_{2}}\,C{{H}_{2}}\,C{{H}_{2}}\,N{{H}_{2}})\]
done
clear
View Answer play_arrow
-
question_answer53) The correct order of increasing thermal stability of \[{{K}_{2}}C{{O}_{3}},\,MgC{{O}_{3}},\,CaC{{O}_{3}}\] and \[BeC{{O}_{3}}\] is:
A)
\[BeC{{O}_{3}}<MgC{{O}_{3}}<{{K}_{2}}C{{O}_{3}}<CaC{{O}_{3}}\]
done
clear
B)
\[BeC{{O}_{3}}<MgC{{O}_{3}}<CaC{{O}_{3}}<{{K}_{2}}C{{O}_{3}}\]
done
clear
C)
\[MgC{{O}_{3}}<BeC{{O}_{3}}<CaC{{O}_{3}}<{{K}_{2}}C{{O}_{3}}\]
done
clear
D)
\[{{K}_{2}}C{{O}_{3}}<MgC{{O}_{3}}<CaC{{O}_{3}}<BeC{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer54) A weak acid, HA, has a \[{{K}_{a}}\] of \[1.00\times {{10}^{-5}}\]. If 0.100 mole of this acid is dissolved in one litre of water, the percentage of acid dissociated at equilibrium is closest to:
A)
99.0%
done
clear
B)
1.00%
done
clear
C)
99.9%
done
clear
D)
0.100%
done
clear
View Answer play_arrow
-
question_answer55) The number of moles of \[KMn{{O}_{4}}\] that will be needed to react with one mole of sulphite ion in acidic solution is:
A)
\[\frac{3}{5}\]
done
clear
B)
\[\frac{4}{5}\]
done
clear
C)
\[\frac{2}{5}\]
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer56) Identify the incorrect statement among the following:
A)
There is a decrease in the radii of die atoms or ions as one proceeds from La to Lu.
done
clear
B)
Lanthanoid contraction is the accumulation of successive shrinkages
done
clear
C)
As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with die 5d series of elements
done
clear
D)
Shielding power of 4f electrons is quite weak.
done
clear
View Answer play_arrow
-
question_answer57) Which of die following oxidation states are die most characteristics for lead and tin respectively?
A)
+4, +2
done
clear
B)
+2, +4
done
clear
C)
+4, +4
done
clear
D)
+2, +2
done
clear
View Answer play_arrow
-
question_answer58) The correct order of C ?O bond length among CO, \[CO_{3}^{2-}\], CO2 is:
A)
CO2 < \[CO_{3}^{2-}\] < CO
done
clear
B)
CO < \[CO_{3}^{2-}\]< CO2
done
clear
C)
\[CO_{3}^{2-}\]< CO2 < CO
done
clear
D)
CO < CI2 < \[CO_{3}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer59) Which of die following represents the correct order of the acidity in the given compounds?
A)
\[C{{H}_{3}}COOH>BrC{{H}_{2}}COOH>ClC{{H}_{2}}COOH>FC{{H}_{2}}COOH\]
done
clear
B)
\[FC{{H}_{2}}COOH>C{{H}_{3}}COOH>BrC{{H}_{2}}COOH>ClC{{H}_{2}}COOH\]
done
clear
C)
\[BrC{{H}_{2}}COOH>Cl\,C{{H}_{2}}\,COOH>FC{{H}_{2}}COOH>C{{H}_{3}}COOH\]
done
clear
D)
\[FC{{H}_{2}}COOH>Cl\,C{{H}_{2}}COOH>BrC{{H}_{2}}COOH>C{{H}_{3}}\,COOH\]
done
clear
View Answer play_arrow
-
question_answer60) The following equilibrium constants are given: \[{{N}_{2}}+3{{H}_{2}}\,\rightleftharpoons \,2N{{H}_{3}}\,;\,{{K}_{1}}\] \[{{N}_{2}}+{{O}_{2}}\,\,\rightleftharpoons \,2NO\,;\,{{K}_{2}}\] \[{{H}_{2}}+1/2\,{{O}_{2}}\,\rightleftharpoons \,{{H}_{2}}O\,;\,{{K}_{3}}\] The equilibrium constant for the oxidation of \[N{{H}_{3}}\] by oxygen to give NO is:
A)
\[{{K}_{2}}K_{3}^{3}/{{K}_{1}}\]
done
clear
B)
\[{{K}_{2}}\,K_{3}^{2}/{{K}_{1}}\]
done
clear
C)
\[K_{2}^{2}\,{{K}_{3}}/{{K}_{1}}\]
done
clear
D)
\[{{K}_{1}}\,{{K}_{2}}/{{K}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer61) Which one of the following vitamins is water-soluble?
A)
Vitamin-B
done
clear
B)
Vitamin-E
done
clear
C)
Vitamin-K
done
clear
D)
Vitamin-A
done
clear
View Answer play_arrow
-
question_answer62) If there is no rotation of plane polarized light by a compound in a specific solvent, thought to be chiral, it may mean that:
A)
the compound is certainly a chiral
done
clear
B)
the compound is certainly meso
done
clear
C)
there is no compound in die solvent
done
clear
D)
the compound may be a racemic mixture
done
clear
View Answer play_arrow
-
question_answer63) Consider die following sets of quantum numbers: \[n\] \[l\] \[m\] \[s\] (i) 3 0 0 \[+1/2\] (ii) 2 2 1 \[+1/2\] (iii) 4 3 -2 \[-1/2\] (iv) 1 0 -1 \[-1/2\] (v) 3 2 3 \[+1/2\] Which of the following sets of quantum number is not possible?
A)
ii, iii and iv
done
clear
B)
i, ii, iii and iv
done
clear
C)
ii, iv and v
done
clear
D)
i and iii
done
clear
View Answer play_arrow
-
question_answer64) Which one of the following ions is the most stable in aqueous solution?
A)
\[C{{r}^{3+}}\]
done
clear
B)
\[{{V}^{3+}}\]
done
clear
C)
\[T{{i}^{3+}}\]
done
clear
D)
Mn3+ (At. no. Ti = 22, V - 23, Cr = 24, Mn = 25)
done
clear
View Answer play_arrow
-
question_answer65) Concentrated aqueous sulphuric acid is 98% \[{{H}_{2}}S{{O}_{4}}\] by mass and has a density of \[1.80\,\,g\,m{{L}^{-1}}\]. Volume of acid required to make one litre of 0.1 M \[{{H}_{2}}S{{O}_{4}}\] solution is:
A)
11.10 mL
done
clear
B)
16.65 mL
done
clear
C)
22.20 mL
done
clear
D)
5.55 Ml
done
clear
View Answer play_arrow
-
question_answer66) Which one of the following ionic species has the greatest proton affinity to form stable compound?
A)
\[H{{S}^{-}}\]
done
clear
B)
\[NH_{2}^{-}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
\[{{I}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer67) \[C{{H}_{3}}-CHCl-C{{H}_{2}}-C{{H}_{3}}\] has a chiral centre. Which one of the following represents its R configuration?
A)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ & {{H}_{3}}C-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-Cl \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,H \\ \end{align}\]
done
clear
B)
\[H-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} {{C}_{2}}{{H}_{5}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
C)
\[\begin{align} & \,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ & Cl-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-C{{H}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,H \\ \end{align}\]
done
clear
D)
\[\begin{align} & \,\,\,\,\,\,\,\,C{{H}_{3}} \\ & H-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-Cl \\ & \,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ \end{align}\]
done
clear
View Answer play_arrow
-
question_answer68)
0.5 molal aqueous solution of a weak acid (HX) is 20% ionized. If 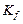 for water is \[1.86\,\,K\,\,kg\,mo{{l}^{-1}},\] the lowering in freezing point of the solution is:
for water is \[1.86\,\,K\,\,kg\,mo{{l}^{-1}},\] the lowering in freezing point of the solution is:
A)
-1.12 K
done
clear
B)
0.56 K
done
clear
C)
1.12 K
done
clear
D)
-0.56K
done
clear
View Answer play_arrow
-
question_answer69) Which one of the following polymers is prepared by condensation polymerization?
A)
Nylon-66
done
clear
B)
Teflon
done
clear
C)
Rubber
done
clear
D)
Styrene
done
clear
View Answer play_arrow
-
question_answer70) The Langmuir adsorption isotherm is deduced using the assumption:
A)
The adsorption takes place in multilayers
done
clear
B)
The adsorption sites are equivalent in their ability to adsorb the particles
done
clear
C)
The heat of adsorption varies with coverage
done
clear
D)
The adsorbed molecules interact with each other
done
clear
View Answer play_arrow
-
question_answer71) The reaction of hydrogen and iodine monochloride is given as: \[{{H}_{2}}(g)+2ICl(g)\xrightarrow{{}}2HCl(g)+{{I}_{2}}(g)\] This reaction is of first order with respect to \[{{H}_{2}}(g)\] and \[ICl(g)\]¸following mechanisms were proposed: Mechanism A: \[{{H}_{2}}(g)+2ICl(g)\xrightarrow{{}}2HCl(g)+{{I}_{2}}(g)\] Mechanism B: \[{{H}_{2}}(g)+ICl(g)\xrightarrow{{}}HCl(g)+HI(g)\]; show \[HI(g)+ICl(g)\xrightarrow{{}}HCl(g)+{{I}_{2}}(g)\]; fast Which of the above mechanism (s) can be consistent with the given information about the reaction?
A)
B only
done
clear
B)
A and B both
done
clear
C)
Neither A nor B
done
clear
D)
A only
done
clear
View Answer play_arrow
-
question_answer72) RNA and DNA are chiral molecules, their chirality is due to:
A)
L-sugar component
done
clear
B)
chiral bases
done
clear
C)
chiral phosphate ester units
done
clear
D)
D-sugar component
done
clear
View Answer play_arrow
-
question_answer73) In which of the following the hydration energy is higher than the lattice energy?
A)
\[BaS{{O}_{4}}\]
done
clear
B)
\[MgS{{O}_{4}}\]
done
clear
C)
\[RaS{{O}_{4}}\]
done
clear
D)
\[SrS{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer74) Which one of the following on reduction with lithium aluminium hydride yield a secondary amine?
A)
Nitroethane
done
clear
B)
Methylisocyanide
done
clear
C)
Acetamide
done
clear
D)
Methyl cyanide
done
clear
View Answer play_arrow
-
question_answer75) Consider the following reactions: (i) \[{{H}^{+}}(aq)+O{{H}^{-}}(aq)={{H}_{2}}O(l)\] \[\Delta H=-{{x}_{1}}\,kJ\,mo{{l}^{-1}}\] (ii) \[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)={{H}_{2}}O(l)\]\[\Delta H=-{{x}_{2}}\,kJ\,mo{{l}^{-1}}\] (iii) \[C{{O}_{2}}(g)+{{H}_{2}}(g)=CO(g)+{{H}_{2}}O(l)\]\[-{{x}_{3}}\,kJ\,mo{{l}^{-1}}\] (iv) \[{{C}_{2}}{{H}_{2}}(g)+\frac{5}{2}{{O}_{2}}(g)=2C{{O}_{2}}(g)+{{H}_{2}}O(l)\]\[+{{x}_{4}}\,kJ\,mo{{l}^{-1}}\] Enthalpy of formation of \[{{H}_{2}}O(l)\] is:
A)
\[-{{x}_{2}}\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[+{{x}_{3}}\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[-{{x}_{4}}\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[-{{x}_{1}}\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer76) Given that bond energies of H-H and CI-CI are \[430\,kJ\,mo{{l}^{-1}}\] and \[240\,kJ\,mo{{l}^{-1}}\] respectively and \[\Delta \]\[{{H}_{f}}\] for HCl is \[-90\,kJ\,mo{{l}^{-1}}\]. Bond enthalpy' of HCl is:
A)
\[290\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[380\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[425\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[245\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer77) Reduction of aldehydes and ketones into hydrocarbons using zinc amalgam and cone. HCl is called:
A)
Clemmensen reduction
done
clear
B)
Cope reduction
done
clear
C)
Dow reduction
done
clear
D)
Wolff-Kishner reduction
done
clear
View Answer play_arrow
-
question_answer78) Which one of the following anions is present in the chain structure silicates?
A)
\[S{{i}_{2}}O_{7}^{6-}\]
done
clear
B)
\[{{(S{{i}_{2}}O_{5}^{2-})}_{n}}\]
done
clear
C)
\[{{(SiO_{3}^{2-})}_{n}}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
-
question_answer79) The fraction of total volume occupied by the atoms present in a simple cube is:
A)
\[\frac{\pi }{6}\]
done
clear
B)
\[\frac{\pi }{3\sqrt{2}}\]
done
clear
C)
\[\frac{\pi }{4\sqrt{2}}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
-
question_answer80) For the following: (i) \[{{I}^{-}}\] (ii) \[C{{l}^{-}}\] (iii) \[B{{r}^{-}}\] the increasing order of nucleophilicity would be:
A)
\[{{I}^{-}}<B{{r}^{-}}<C{{l}^{-}}\]
done
clear
B)
\[C{{l}^{-}}<B{{r}^{-}}<{{I}^{-}}\]
done
clear
C)
\[{{I}^{-}}<C{{l}^{-}}<B{{r}^{-}}\]
done
clear
D)
\[B{{r}^{-}}<C{{l}^{-}}<{{I}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer81) Which one of the following orders correctly represents the increasing acid strengths of the given acids?
A)
\[HOCl<HOClO<HOCl{{O}_{2}}<HOCl{{O}_{3}}\]
done
clear
B)
\[HOClO<HOCl<HOCl{{O}_{3}}<HOCl{{O}_{2}}\]
done
clear
C)
\[HOClO<HOCl<HOCl{{O}_{3}}<HOCl{{O}_{2}}\]
done
clear
D)
\[HOCl{{O}_{2}}<HOCl{{O}_{3}}<HOClO<HOCl\]
done
clear
View Answer play_arrow
-
question_answer82) The reaction: \[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-O-C{{H}_{2}}-C{{H}_{3}}+HI\xrightarrow{Heated}....\] Which of the following compounds will be formed?
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-I+C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}+C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}OH+C{{H}_{3}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}OH+C{{H}_{3}}-C{{H}_{2}}-I\]
done
clear
View Answer play_arrow
-
question_answer83) With which of the following electronic configuration an atom has the lowest ionisation enthalpy?
A)
\[1{{s}^{2}}\,2{{s}^{2}}\,2{{p}^{5}}\]
done
clear
B)
\[1{{s}^{2\,}}\,2{{s}^{2}}\,2{{p}^{3}}\]
done
clear
C)
\[1{{s}^{2}}\,2{{s}^{2}}\,2{{p}^{5}}\,3{{s}^{1}}\]
done
clear
D)
\[1{{s}^{2}}\,2{{s}^{2}}\,2{{p}^{6}}\]
done
clear
View Answer play_arrow
-
question_answer84) Predict the product C obtained in the following reaction of butyne-1. \[C{{H}_{3}}C{{H}_{2}}-C\equiv CH+HCl\xrightarrow[{}]{{}}B\xrightarrow[{}]{HI}C\]
A)
\[\begin{align} & C{{H}_{3}}-\underset{|}{\mathop{C}}\,H-C{{H}_{2}}C{{H}_{2}}I \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,Cl \\ \end{align}\]
done
clear
B)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,I \\ & C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Cl \\ \end{align}\]
done
clear
C)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,I \\ & C{{H}_{3}}-C{{H}_{2}}-\overset{|}{\mathop{C}}\,H-C{{H}_{2}}Cl \\ \end{align}\]
done
clear
D)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,I \\ & C{{H}_{3}}C{{H}_{2}}-\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,-C{{H}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Cl \\ \end{align}\]
done
clear
View Answer play_arrow
-
question_answer85) An element, X has the following isotopic composition; \[^{200}X:90%\] \[^{199}X:8.0%\] \[^{202}X:2.0%\] The weighted average atomic mass of the naturally-occurring element X is closest to:
A)
200 amu
done
clear
B)
201 amu
done
clear
C)
202 amu
done
clear
D)
199 amu
done
clear
View Answer play_arrow
-
question_answer86) In a first order reaction \[A\xrightarrow[{}]{{}}B\], if k is rate constant and initial concentration of the reactant A is 0.5 M then the half-life is:
A)
\[\frac{0.693}{0.5\,k}\]
done
clear
B)
\[\frac{\log \,2}{k}\]
done
clear
C)
\[\frac{\log \,2}{k\sqrt{0.5}}\]
done
clear
D)
\[\frac{\ln \,2}{k}\]
done
clear
View Answer play_arrow
-
question_answer87) Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
A)
Carbon and hydrogen are suitable reducing agents for metal sulphides
done
clear
B)
The \[{{\Delta }_{f}}{{G}^{o}}\] of the sulphide is greater than those for \[C{{S}_{2}}\] and \[{{H}_{2}}S\]
done
clear
C)
The \[{{\Delta }_{f}}{{G}^{o}}\] is negative for roasting of sulphide ore to oxide
done
clear
D)
Roasting of the sulphide to the oxide is thermodynamicaliy feasible
done
clear
View Answer play_arrow
-
question_answer88) If 60% of a first order reaction was completed in 60 min, 50% of the same reaction would be completed in approximately:
A)
50 min
done
clear
B)
45 min
done
clear
C)
60 min
done
clear
D)
40 min (log 4 = 0.60, log 5 = 0.69)
done
clear
View Answer play_arrow
-
question_answer89) The equilibrium constant of the reaction: \[Cu(s)\,+2A{{g}^{+}}(aq)\,\to C{{u}^{2+}}(aq)+2Ag(s);\] \[{{E}^{o}}=0.46\,V\] at 298 K is:
A)
\[2.4\times {{10}^{10}}\]
done
clear
B)
\[2.0\times {{10}^{10}}\]
done
clear
C)
\[4.0\times {{10}^{10}}\]
done
clear
D)
\[4.0\times {{10}^{15}}\]
done
clear
View Answer play_arrow
-
question_answer90) Which of the compounds with molecular formula \[{{C}_{5}}{{H}_{10}}\] yields acetone on ozonolysis?
A)
2-methyl-2-butene
done
clear
B)
3-methyl-l-butene
done
clear
C)
Cyclopentane
done
clear
D)
2-methyl-l-butene
done
clear
View Answer play_arrow
-
question_answer91) Sulphide ores of metals are usually concentrated by froth flotation process. Which one of the following sulphide ores offers an exception and is concentrated by chemical leaching?
A)
Argentite
done
clear
B)
Galena
done
clear
C)
Copper pyrite
done
clear
D)
Sphalerite
done
clear
View Answer play_arrow
-
question_answer92) The efficiency of a fuel cell is given by:
A)
\[\frac{\Delta H}{\Delta G}\]
done
clear
B)
\[\frac{\Delta G}{\Delta S}\]
done
clear
C)
\[\frac{\Delta G}{\Delta H}\]
done
clear
D)
\[\frac{\Delta S}{\Delta G}\]
done
clear
View Answer play_arrow
-
question_answer93) Which one of the following on treatment with 50% aqueous sodium hydroxide yields the corresponding alcohol and acid?
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
D)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O \\ & C{{H}_{3}}-\overset{||}{\mathop{C}}\,-C{{H}_{3}} \\ \end{align}\]
done
clear
View Answer play_arrow
-
question_answer94) Identify the correct order of the size of the following:
A)
\[C{{a}^{2+}}<{{K}^{+}}<Ar<{{S}^{2-}}<C{{l}^{-}}\]
done
clear
B)
\[C{{a}^{2+}}<{{K}^{+}}<Ar<C{{l}^{-}}<{{S}^{2-}}\]
done
clear
C)
\[Ar<C{{a}^{2+}}<{{K}^{+}}<C{{l}^{-}}<{{S}^{2-}}\]
done
clear
D)
\[C{{a}^{2+}}<Ar<{{K}^{+}}<C{{l}^{-}}<{{S}^{2-}}\]
done
clear
View Answer play_arrow
-
A)
(ii) > (iv) > (iii) > (i)
done
clear
B)
(i) > (ii) > (iii) > (iv)
done
clear
C)
(iv) > (ii) > (i) > (iii)
done
clear
D)
(ii) > (iv) > (i) > (iii)
done
clear
View Answer play_arrow
-
question_answer96) The product formed in aldol condensation is:
A)
a beta-hydroxy acid
done
clear
B)
a beta-hydroxy aldehyde or a beta-hydroxy ketone
done
clear
C)
an alpha-hydroxy aldehyde or ketone
done
clear
D)
an alpha, beta unsaturated ester
done
clear
View Answer play_arrow
-
question_answer97) If NaCl is doped with 10-4 mol % of \[SrC{{l}_{2}},\] the concentration of cation vacancies will be \[({{N}_{A}}=6.02\,\times {{10}^{23}}\,mo{{l}^{-1}}):\]
A)
\[6.02\,\times {{10}^{15}}\,mo{{l}^{-1}}\]
done
clear
B)
\[6.02\,\times {{10}^{16}}\,mo{{l}^{-1}}\]
done
clear
C)
\[6.02\,\times {{10}^{17}}\,mo{{l}^{-1}}\]
done
clear
D)
\[6.02\,\times {{10}^{14}}\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer98) The d-electron configurations of \[C{{r}^{2+}},\,M{{n}^{2+}},\,F{{e}^{2+}}\] and \[N{{i}^{2+}}\] are \[3{{d}^{4}},\,3{{d}^{5}},\,3{{d}^{6}}\] and \[3{{d}^{8}}\] respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
A)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\] (At. no. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
done
clear
View Answer play_arrow
-
question_answer99) The order of decreasing reactivity towards an electrophilic reagent, for the following: (i) Benzene (ii) Toluene (iii) Chlorobenzene and (iv) Phenol would be:
A)
(i) > (ii) > (iii) > (iv)
done
clear
B)
(ii) > (iv) > (i) > (iii)
done
clear
C)
(iv) > (iii) > (ii) > (i)
done
clear
D)
(iv) > (ii) > (i) > (iii)
done
clear
View Answer play_arrow
-
question_answer100) In which of the following pairs, the two species are iso-structural?
A)
\[S{{F}_{4}}\] and \[Xe{{F}_{4}}\]
done
clear
B)
\[SO_{3}^{2-}\] and \[NO_{3}^{-}\]
done
clear
C)
\[B{{F}_{3}}\] and \[N{{F}_{3}}\]
done
clear
D)
\[BrO_{3}^{-}\] and \[Xe{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer101) Passage cells are thin-walled cells found in:
A)
endodermis of roots facilitating rapid transport of water from cortex to pericycle
done
clear
B)
phloem elements that serve as entry points for substances for transport to other plant parts
done
clear
C)
testa of seeds to enable emergence of growing embryonic axis during seed germination
done
clear
D)
central region of style through which the pollen tube grows towards the ovary
done
clear
View Answer play_arrow
-
question_answer102) Opening of floral buds into flowers, is a type of:
A)
autonomic movement of locomotion
done
clear
B)
autonomic movement of variation
done
clear
C)
paratonic movement of growth
done
clear
D)
autonomic movement of growth
done
clear
View Answer play_arrow
-
question_answer103) Telomere repetitive DNA sequences control the function of eukaryotic chromosomes because they:
A)
act as replicons
done
clear
B)
are RNA transcription initiator
done
clear
C)
help chromosome pairing
done
clear
D)
prevent chromosome loss
done
clear
View Answer play_arrow
-
question_answer104) Identify the odd combination of the habitat and the particular animal concerned:
A)
Dachigam National Park? Snow Leopard
done
clear
B)
Sunderbans?Bengal Tiger
done
clear
C)
Periyar?Elephant
done
clear
D)
Rann of Kutch?Wild Ass
done
clear
View Answer play_arrow
-
question_answer105) In the leaves of \[{{C}_{4}}\] plants, malic acid formation during \[C{{O}_{2}}\] fixation occurs in the cells of:
A)
mesophyll
done
clear
B)
bundle sheath
done
clear
C)
phloem
done
clear
D)
epidermis
done
clear
View Answer play_arrow
-
question_answer106) A human male produces sperms with the genotypes AB, Ah, aB and ab pertaining to two diallelic characters in equal proportions. What is the corresponding genotype of this person?
A)
AaBb
done
clear
B)
AaBB
done
clear
C)
AABb
done
clear
D)
AABB
done
clear
View Answer play_arrow
-
question_answer107) Which of the following ecosystem types has the highest annual net primary productivity?
A)
Tropical rain forest
done
clear
B)
Tropical deciduous forest
done
clear
C)
Temperate evergreen forest
done
clear
D)
Temperate deciduous forest
done
clear
View Answer play_arrow
-
question_answer108) In human body, which one of the following is anatomically correct?
A)
Floating ribs?2 pairs
done
clear
B)
Collar bones?3 pairs
done
clear
C)
Salivary glands?1 pair
done
clear
D)
Cranial nerves?10 pairs
done
clear
View Answer play_arrow
-
question_answer109) Which of the following is a flowering plant with nodules containing filamentous nitrogen-fixing microorganism?
A)
Casuarina equisetifolia
done
clear
B)
Crotolaria juncea
done
clear
C)
Cycas revolute
done
clear
D)
Cicer arietinum
done
clear
View Answer play_arrow
-
question_answer110) The population of an insect species shows an explosive increase in numbers during rainy season followed by its disappearance at the end of the season. What does this show?
A)
S-shaped or sigmoid growth of this insect
done
clear
B)
The food plants mature and die at the end of the rainy season
done
clear
C)
Its population growth curve is of J-type
done
clear
D)
The population of its predators increases enormously
done
clear
View Answer play_arrow
-
question_answer111) One of endangered species of Indian medicinal plants is that of:
A)
Podophyllum
done
clear
B)
Ocimum
done
clear
C)
Garlic
done
clear
D)
Nepenthes
done
clear
View Answer play_arrow
-
question_answer112) In the hexaploid wheat, the haploid (n) and basic (x) numbers of chromosomes are:
A)
\[x=7\] and \[x=21\]
done
clear
B)
\[n=21\] and \[x=21\]
done
clear
C)
\[n=21\] and \[x=14\]
done
clear
D)
\[n=21\] and \[x=7\]
done
clear
View Answer play_arrow
-
question_answer113) In cloning of cattle a fertilized egg is taken out of the mother's womb band:
A)
the egg is divided into 4 pairs of cells which are implanted into the womb of other cows
done
clear
B)
in the eight cell stage, cells are separated and cultured until small embryos are formed which are implanted into the womb of other cows
done
clear
C)
in the eight cell stage the individual cells are separated under electrical field for further development in culture media
done
clear
D)
from this upto eight identical twins can be produced
done
clear
View Answer play_arrow
-
question_answer114) Which one of the following statements is correct?
A)
Extensive use of chemical fertilizers may lead to eutrophication of nearby water bodies
done
clear
B)
Both Azotobacter and Rhizobium fix atmospheric nitrogen in root nodules of plants
done
clear
C)
Cyanobacteria such as Anabaena and Nostoc are important mobilizers of phosphates and potassium for plant nutrition in soil
done
clear
D)
At present it is not possible to grow maize without chemical fertilizers
done
clear
View Answer play_arrow
-
question_answer115) One of the important consequences of geographical isolation is:
A)
no change in the isolated fauna
done
clear
B)
preventing speciation
done
clear
C)
speciation through reproductive isolation
done
clear
D)
random creation of new species
done
clear
View Answer play_arrow
-
question_answer116) Flagellated male gametes are present in all die three of which one of the following sets?
A)
Anthoceros, Funaria and Spirogyra
done
clear
B)
Zygnema, Saprolegnia and Hydrilla
done
clear
C)
Fucus, Marsilea and Calotropis
done
clear
D)
Riccia, Dryopteris and Cycas
done
clear
View Answer play_arrow
-
question_answer117) Molecular basis of organ differentiation depends on the modulation in transcription by:
A)
RNA polymerase
done
clear
B)
ribosome
done
clear
C)
transcription factor
done
clear
D)
anticodon
done
clear
View Answer play_arrow
-
question_answer118) Increased asthmatic attacks in certain seasons are related to:
A)
hot and humid environment
done
clear
B)
eating fruits preserved in tin containers
done
clear
C)
inhalation of seasonal pollen
done
clear
D)
low temperature
done
clear
View Answer play_arrow
-
question_answer119) Which of the following is a slime mould?
A)
Rhizopus
done
clear
B)
Physarum
done
clear
C)
Thiobacillus
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
-
question_answer120) Which one of the following is an example of negative feedback loop in humans?
A)
Constriction of skin blood vessels and contraction of skeletal muscles when it is too cold
done
clear
B)
Secretion of tears after falling of sand particles into the eye
done
clear
C)
Salivation of mouth at the sight of delicious food
done
clear
D)
Secretion of sweat glands and constriction of skin blood vessels when it is too hot
done
clear
View Answer play_arrow
-
question_answer121) Ergot of rye is caused by a species of:
A)
Phytophthora
done
clear
B)
Uncinula
done
clear
C)
Ustilago
done
clear
D)
Claviceps
done
clear
View Answer play_arrow
-
question_answer122) Geometric representation of age structure is a characteristic of:
A)
Biotic community
done
clear
B)
Population
done
clear
C)
Landscape
done
clear
D)
Ecosystem
done
clear
View Answer play_arrow
-
question_answer123) Which one of the following mammalian cells is not capable of metabolising glucose to carbon-dioxide aerobically?
A)
White blood cells
done
clear
B)
Unstriated muscle cells
done
clear
C)
Liver cells
done
clear
D)
Red blood cells
done
clear
View Answer play_arrow
-
question_answer124) Feeling the tremors of an earthquake a scared resident of seventh floor of a multistoryed building starts climbing down the stairs rapidly. Which hormone initiated this action?
A)
Thyroxin
done
clear
B)
Adrenaline
done
clear
C)
Glucagon
done
clear
D)
Gastrin
done
clear
View Answer play_arrow
-
question_answer125) Among the human ancestors the brain size was more than 1000 CC in:
A)
Homo neaderthalensis
done
clear
B)
Homo erectus
done
clear
C)
Ramapithecus
done
clear
D)
Homo habilis
done
clear
View Answer play_arrow
-
question_answer126) The length of DNA molecule greatly exceeds the dimensions of the nucleus in eukaryotic cells. How is this DMA accommodated?
A)
Deletion of non-essential genes
done
clear
B)
Super-coiling in nucleosomes
done
clear
C)
DNAse digestion
done
clear
D)
Through elimination of repetitive DNA
done
clear
View Answer play_arrow
-
question_answer127) The wavelength of light absorbed by \[{{P}_{r}}\] form of phytochrome is:
A)
640 nm
done
clear
B)
680 nm
done
clear
C)
720 nm
done
clear
D)
620 nm
done
clear
View Answer play_arrow
-
question_answer128) Which part of ovary in mammals acts as an endocrine gland after ovulation?
A)
Graaffian follicle
done
clear
B)
Stroma
done
clear
C)
Germinal epithelium
done
clear
D)
Vitelline membrane
done
clear
View Answer play_arrow
-
question_answer129) Which one of the following is not a constituent of cell membrane?
A)
Cholesterol
done
clear
B)
Glycolipids
done
clear
C)
Proline
done
clear
D)
Phospholipids
done
clear
View Answer play_arrow
-
question_answer130) The concept of chemical evolution is based on:
A)
crystallization of chemicals
done
clear
B)
interaction of water, air and clay under intense heat
done
clear
C)
effect of solar radiation on chemicals
done
clear
D)
possible origin of life by combination of chemicals under suitable environmental conditions
done
clear
View Answer play_arrow
-
question_answer131) In maize, hybrid vigour is exploited by:
A)
bombarding the seeds with DNA
done
clear
B)
crossing of two inbred parental lines
done
clear
C)
harvesting seeds from the most productive plants
done
clear
D)
inducing mutations
done
clear
View Answer play_arrow
-
question_answer132) In gymnosperms, die pollen chamber represents:
A)
a cell in the pollen grain in which the sperms are formed
done
clear
B)
a cavity in the ovule in which pollen grains are stored after pollination
done
clear
C)
an opening in the megagametophyte through which the pollen tube approaches die egg
done
clear
D)
the microsporangium in which pollen grains develop
done
clear
View Answer play_arrow
-
question_answer133) During transcription, RNA polymerase holoenzyme binds to a gene promoter and assumes a saddle-like structure. What is it's DNA-binding sequence?
A)
TTAA
done
clear
B)
AATT
done
clear
C)
CACC
done
clear
D)
TATA
done
clear
View Answer play_arrow
-
question_answer134) In die prothallus of a vascular cryptogam, die antherozoids and eggs mature at different times. As a result:
A)
there is no change in success rate of fertilization
done
clear
B)
there is high degree of sterility
done
clear
C)
one can conclude that die plant is apomictic
done
clear
D)
self fertilization is prevented
done
clear
View Answer play_arrow
-
question_answer135) Industrial melanism as observed in peppered moth proves that:
A)
The true black melanic forms arise by a recurring random mutation
done
clear
B)
The melanic form of the moth has no selective advantage over lighter form in industrial area
done
clear
C)
The lighter-form moth has no selective advantage either in polluted industrial area or non-polluted area
done
clear
D)
Melanism is a pollution- generated feature
done
clear
View Answer play_arrow
-
question_answer136) ?Foolish seedling? disease of rice led to die discover of:
A)
GA
done
clear
B)
ABA
done
clear
C)
2, 4-D
done
clear
D)
1AA
done
clear
View Answer play_arrow
-
question_answer137) Differentiation of organs and tissues in a developing organism, is associated with:
A)
developmental mutations
done
clear
B)
differential expression of genes
done
clear
C)
lethal mutations
done
clear
D)
deletion of gens
done
clear
View Answer play_arrow
-
question_answer138) The first acceptor of electrons from an excited chlorophyll molecule of photosystem II is:
A)
cytochrome
done
clear
B)
iron-sulphur protein
done
clear
C)
ferredoxin
done
clear
D)
quinine
done
clear
View Answer play_arrow
-
question_answer139) Lysozyme that is present in perspiration, saliva and tears, destroys:
A)
certain fungi
done
clear
B)
certain types of bacteria
done
clear
C)
all viruses
done
clear
D)
most virus-infected cells
done
clear
View Answer play_arrow
-
question_answer140) During the transmission of nerve impulse through a nerve fibre, the potential on the inner side of the plasma membrane has which type of electric charge?
A)
First negative, then positive and again back to negative
done
clear
B)
First positive, then negative and continue to be negative
done
clear
C)
First negative, then positive and continue to be positive
done
clear
D)
First positive, then negative and again back to positive
done
clear
View Answer play_arrow
-
question_answer141) The Okazaki fragments in DNA chain growth:
A)
result in transcription
done
clear
B)
polymerize in the 3'-to-5' direction and forms replication fork
done
clear
C)
prove semi-conservative nature of DNA replication
done
clear
D)
polymerize in the 5'-to-3' direction and explain 3'-to-5' DNA replication
done
clear
View Answer play_arrow
-
question_answer142) The two polynucleotide chains in DNA are:
A)
parallel
done
clear
B)
discontinuous
done
clear
C)
antiparallel
done
clear
D)
semiconservative
done
clear
View Answer play_arrow
-
question_answer143) Which one of the following is a viral disease of poultry?
A)
Salmonellosis
done
clear
B)
Coryza
done
clear
C)
New castle disease
done
clear
D)
Pasteurellosis
done
clear
View Answer play_arrow
-
question_answer144) Inheritance of skin colour in humans is an example of:
A)
chromosomal aberration
done
clear
B)
point mutation
done
clear
C)
polygenic inheritance
done
clear
D)
codominance
done
clear
View Answer play_arrow
-
question_answer145) One gene-one enzyme relationship was established for the first time in:
A)
Neurospora crassa
done
clear
B)
Salmonella typhimurium
done
clear
C)
Escherichia coli
done
clear
D)
Diplococcus pneumonia
done
clear
View Answer play_arrow
-
question_answer146) A genetically engineered micro-organism used successfully in bioremediation of oil spills is a species of:
A)
Pseudomonas
done
clear
B)
Trichoderma
done
clear
C)
Xanthomonas
done
clear
D)
Bacillus
done
clear
View Answer play_arrow
-
question_answer147) In which one of the following preparations are you likely to come across cell junctions most frequently?
A)
Ciliated epithelium
done
clear
B)
Thrombocytes
done
clear
C)
Tendon
done
clear
D)
Hyaline cartilage
done
clear
View Answer play_arrow
-
question_answer148) Which one of the following pairs is mismathced?
A)
Pila globosa ? pearl
done
clear
B)
Apis indica ? honey
done
clear
C)
Kenia lacca ? lac
done
clear
D)
Bombyx mori ? silk
done
clear
View Answer play_arrow
-
question_answer149) About 98 percent of the mass of every living organism is composed of just six elements including carbon, hydrogen, nitrogen, oxygen and:
A)
phosphorus and sulphur
done
clear
B)
sulphur and magnesium
done
clear
C)
magnesium and sodium
done
clear
D)
calcium and phosphorus
done
clear
View Answer play_arrow
-
question_answer150) Two genes R and Y are located very close on the chromosomal linkage map of maize plant. When RRYV and rryy genotypes are hybridized, then \[{{F}_{2}}\] segregation will show:
A)
higher number of the recombinant types
done
clear
B)
segregation in the expected 9 : 3 : 3 : 1 ratio
done
clear
C)
segregation in 3 : 1 ratio
done
clear
D)
higher number of the parental types
done
clear
View Answer play_arrow
-
question_answer151) What is common between parrot, platypus and kangaroo?
A)
Homeothermy
done
clear
B)
Toothless jaws
done
clear
C)
Functional post-anal tail
done
clear
D)
Ovopariry
done
clear
View Answer play_arrow
-
question_answer152) Which one of the following is being utilized as a source of bio- diesel in the Indian countryside?
A)
Euphorbia
done
clear
B)
Beetroot
done
clear
C)
Sugarcane
done
clear
D)
Pongamia
done
clear
View Answer play_arrow
-
question_answer153) In which one of the following the BOD (Biochemical Oxygen Demand) of sewage (S), distillery effluent (DE), paper mill effluent (PE) and sugar mill effluent (SE) have been arranged in ascending order ?
A)
SE < S < PE < DE
done
clear
B)
SE < PE < S < DE
done
clear
C)
PE < S < SE < DE
done
clear
D)
S < DE < PE < SE
done
clear
View Answer play_arrow
-
question_answer154) In the human female, menstruation can be deferred by the administration of:
A)
LH only
done
clear
B)
Combination of FSH and LH
done
clear
C)
Combination of estrogen and progesterone
done
clear
D)
FSH only
done
clear
View Answer play_arrow
-
question_answer155) Select the correct statement from the following:
A)
Darwinian variations are small and directionless
done
clear
B)
fitness is the end result of the ability to adapt and gets selected by nature
done
clear
C)
all mammals except whales and camels have seven cervical vertebrae
done
clear
D)
mutations are random and directional
done
clear
View Answer play_arrow
-
question_answer156) Two plants can be conclusively said to belong to the same species if they:
A)
can reproduce freely with each other and form seeds
done
clear
B)
have more than 90 per cent similar genes
done
clear
C)
look similar and possess identical secondary metabolites
done
clear
D)
have same number of chromosomes
done
clear
View Answer play_arrow
-
question_answer157) A sequential expression of a set of human genes occurs when a steroid molecule binds to the:
A)
transfer RNA
done
clear
B)
messenger RNA
done
clear
C)
DNA sequence
done
clear
D)
ribosome
done
clear
View Answer play_arrow
-
question_answer158) In a coal fired power plant electrostatic precipitators are installed to control emission of:
A)
\[S{{O}_{2}}\]
done
clear
B)
\[N{{O}_{x}}\]
done
clear
C)
SPM
done
clear
D)
CO
done
clear
View Answer play_arrow
-
question_answer159) Probiotics are:
A)
safe antibiotics
done
clear
B)
cancer inducing microbes
done
clear
C)
new kind of food allergens
done
clear
D)
live microbial food supplement
done
clear
View Answer play_arrow
-
question_answer160) Which pair of the following belongs to Basidiomycetes?
A)
Birds nest fungi and Pufballs
done
clear
B)
Pufballs and Claviceps
done
clear
C)
Peziza and Stink horns
done
clear
D)
Morchella and Mushrooms
done
clear
View Answer play_arrow
-
question_answer161) Spore dissemination in some liverworts is aided by:
A)
elaters
done
clear
B)
indusium
done
clear
C)
calyptra
done
clear
D)
peristome teeth
done
clear
View Answer play_arrow
-
question_answer162) A person who is on a long hunger strike and is surviving only on water, will have:
A)
more sodium in his urine
done
clear
B)
less amino acids in his urine
done
clear
C)
more glucose in his blood
done
clear
D)
less urea in his urine
done
clear
View Answer play_arrow
-
question_answer163) Which one of the following pairs, is not correctly matched?
A)
Abscisic acid ? Stomatal closure
done
clear
B)
Gibberellic acid ?Leaf fall
done
clear
C)
Cytokinin ?Cell division
done
clear
D)
IAA ? Cell wall elongation
done
clear
View Answer play_arrow
-
question_answer164) A common test to find the genotype of a hybrid is by:
A)
crossing of one \[{{F}_{2}}\] progeny with male parent
done
clear
B)
crossing of one \[{{F}_{2}}\] progeny with female parent
done
clear
C)
studying the sexual behaviour of \[{{F}_{1}}\] progenies
done
clear
D)
crossing of one \[{{F}_{1}}\] progeny with male parent
done
clear
View Answer play_arrow
-
question_answer165) Which one of the following is a matching pair of a body feature and the animal possessing it?
A)
Post-anal tail ? Octopus
done
clear
B)
Ventral Central ? Leech nervous system
done
clear
C)
Pharyngeal gills slits ? Chamaeleon absent in embryo
done
clear
D)
Ventral heart ? Scorpion
done
clear
View Answer play_arrow
-
question_answer166) Which of the following pairs are correctly matched?
A)
Animals Morphological features A. Crocodile ? 4-chambered heart B. Sea urchin ? Parapodia C. Obelia ? Metagenesis D. Lemur ? Thecodont A, C and D
done
clear
B)
B, C and D
done
clear
C)
Only A and D
done
clear
D)
Only A and B
done
clear
View Answer play_arrow
-
question_answer167) If you suspect major deficiency of antibodies in a person, to which of the following would you look for confirmatory evidence?
A)
Serum albumins
done
clear
B)
Serum globulins
done
clear
C)
Fibrinogen in the plasma
done
clear
D)
Haemocytes
done
clear
View Answer play_arrow
-
question_answer168) A person is having problems with calcium and phosphorus metabolism in his body. Which one of the following glands may not be functioning properly?
A)
Parathyroid
done
clear
B)
Parotid
done
clear
C)
Pancreas
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
-
question_answer169) What is true about Nereis, Scorpion, Cockroach and Silver fish?
A)
They all have jointed paired appendages
done
clear
B)
They all possess dorsal heart
done
clear
C)
None of them is aquatic
done
clear
D)
They all belong to the same phylum
done
clear
View Answer play_arrow
-
question_answer170) The finches of Galapagos islands provide an evidence in favour of:
A)
special creation
done
clear
B)
evolution due to mutation
done
clear
C)
retrogressive evolution
done
clear
D)
biogeographical evolution
done
clear
View Answer play_arrow
-
question_answer171) Two cells A and B are contiguous. Cell A has osmotic pressure 10 atm, turgor pressure-7 atm and diffusion pressure deficit 3 atm. Cell B has osmotic pressure 8 atm, turgor pressure 3 atm and diffusion pressure deficit 5 atm. The result will be:
A)
Movement of water from cell B to A
done
clear
B)
No movement of water
done
clear
C)
Equilibrium between the two
done
clear
D)
Movement of water from cell A to B
done
clear
View Answer play_arrow
-
question_answer172) Which one of the following elements is not an essential micronutrient for plant growth?
A)
Mn
done
clear
B)
Zn
done
clear
C)
Cu
done
clear
D)
Ca
done
clear
View Answer play_arrow
-
question_answer173) Male gametes in angiosperms are formed by the division of:
A)
microspore
done
clear
B)
generative cell
done
clear
C)
vegetative cell
done
clear
D)
microspore mother cell
done
clear
View Answer play_arrow
-
question_answer174) Which one of the following is surrounded by a callose wall?
A)
Microspore mother cell
done
clear
B)
Male gemete
done
clear
C)
Egg
done
clear
D)
Pollen grain
done
clear
View Answer play_arrow
-
question_answer175) Which one of the following statements about mycoplasma is wrong?
A)
They are also called PPLO
done
clear
B)
They are pleomorphic
done
clear
C)
They are sensitive to penicillin
done
clear
D)
They cause disease in plants
done
clear
View Answer play_arrow
-
question_answer176) Which one of the following is not a bioindicator of water pollution?
A)
Sludge-worms
done
clear
B)
Blood-worms
done
clear
C)
Stone files
done
clear
D)
Sewage fungus
done
clear
View Answer play_arrow
-
question_answer177) Select the wrong statement from the following:
A)
both chloroplasts and mitochondria contain an inner and an outer membrane
done
clear
B)
both chloroplasts and mitochondria have an internal compartment, the thylakoid space bounded by the thylakoid membrane
done
clear
C)
both chloroplasts and mitochondria contain DNA
done
clear
D)
the chloroplasts are generally much larger than mitochondria
done
clear
View Answer play_arrow
-
question_answer178) Which one of the following pairs of organisms are exotic species introduced in India?
A)
Ficus religiosa, Lantana camara
done
clear
B)
Lantana camara, Water hyacinth
done
clear
C)
Water hyacinth, Prosopis cineraria
done
clear
D)
Nile perch, Fiats religiosa
done
clear
View Answer play_arrow
-
question_answer179) Which one of the following statement is correct?
A)
Stem cells are specialized cells
done
clear
B)
There is no evidence of the existence of gills during embryogenesis of mammals
done
clear
C)
All plant and animal cells are totipotent
done
clear
D)
Ontogeny repeats phylogeny
done
clear
View Answer play_arrow
-
question_answer180) Bowman's glands are located in the:
A)
proximal end of uriniferous tubules
done
clear
B)
anterior pituitary
done
clear
C)
female reproductive system of cockroach
done
clear
D)
olfactory epithelium of our nose
done
clear
View Answer play_arrow
-
question_answer181) The living organisms can be unexceptionally distinguished from the non-living things on the basis of their ability for:
A)
responsiveness to touch
done
clear
B)
interaction with the environment and progressive evolution
done
clear
C)
reproduction
done
clear
D)
growth and movement
done
clear
View Answer play_arrow
-
question_answer182) Compared to a bull a bullock is docile because of:
A)
higher levels of thyroxin
done
clear
B)
higher levels of cortisone
done
clear
C)
lower levels of blood testosterone
done
clear
D)
lower levels of adrenalin/ noradrenalin in its blood
done
clear
View Answer play_arrow
-
question_answer183) What is common to whale, seal and shark?
A)
Seasonal migration
done
clear
B)
Thick subcutaneous fat
done
clear
C)
Convergent evolution
done
clear
D)
Homeothermy
done
clear
View Answer play_arrow
-
question_answer184) All enzymes of TCA cycle are located in the mitochondrial matrix except one which is located in inner mitochondrial membranes in eukaryotes and in cytosol in prokaryotes. This enzyme is:
A)
lactate dehydrogenase
done
clear
B)
isocitrate dehydrogenase
done
clear
C)
malate dehydrogenase
done
clear
D)
succinate dehydrogenase
done
clear
View Answer play_arrow
-
question_answer185) Which one of the following pairs of structures distinguishes a nerve cell from other types of cell?
A)
Perikaryon and dendrites
done
clear
B)
Vacuoles and fibres
done
clear
C)
Flagellum and medullary sheath
done
clear
D)
Nucleus and mitochondria
done
clear
View Answer play_arrow
-
question_answer186) Ultrasound of how much frequency is beamed into human body for sonography?
A)
30-45 MHz
done
clear
B)
15-30 MHz
done
clear
C)
1-15 MHz
done
clear
D)
45-70 MHz
done
clear
View Answer play_arrow
-
question_answer187) Biological organisation starts with:
A)
Submicroscopic molecular level
done
clear
B)
Cellular level
done
clear
C)
Organismic level
done
clear
D)
Atomic level
done
clear
View Answer play_arrow
-
question_answer188) Which one of the following pairs is wrongly matched?
A)
Methanogens ? Gobar gas
done
clear
B)
Yeast ? Ethanol
done
clear
C)
Streptomycetes ? Antibiotic
done
clear
D)
Coliforms ? Vinegar
done
clear
View Answer play_arrow
-
question_answer189) In pea plants, yellow seeds are dominant to green. If a heterozygous yellow seeded plant is crossed with a green seeded plant, what ratio of yellow and green seeded plants would you expect in F1 generation?
A)
50 : 50
done
clear
B)
9 : 1
done
clear
C)
1 : 3
done
clear
D)
3 : 1
done
clear
View Answer play_arrow
-
question_answer190) Adaptive radiation refers to:
A)
adaptations due to geographical isolation
done
clear
B)
evolution of different species from a common ancestor
done
clear
C)
migration of members of a species to different geographical areas
done
clear
D)
power of adaptation in an individual to a variety of environments
done
clear
View Answer play_arrow
-
question_answer191) If the mean and the median pertaining to a certain character of a population are of the same value, the following is most likely to occur:
A)
a normal distribution
done
clear
B)
a bi-modal distribution
done
clear
C)
a T-shaped curve
done
clear
D)
a skewed curve
done
clear
View Answer play_arrow
-
question_answer192) When two species of different genealogy come to resemble each other as a result of adaptation, the phenomenon is termed:
A)
divergent evolution
done
clear
B)
microevolurion
done
clear
C)
co-evolution
done
clear
D)
convergent evolution
done
clear
View Answer play_arrow
-
question_answer193) Which one of the following is a fat-soluble vitamin and its related deficiency disease?
A)
Ascorbic acid ? Scurvy
done
clear
B)
Retinol ? Xerophthalmia
done
clear
C)
Cobalamine ? Beri-beri
done
clear
D)
Calciferol ? Pellagra
done
clear
View Answer play_arrow
-
question_answer194) ICBN stands for:
A)
Indian Congress of Biological Names
done
clear
B)
International Code of Botanical Nomenclature
done
clear
C)
International Congress of Biological Names
done
clear
D)
Indian Code of Botanical Nomenclature
done
clear
View Answer play_arrow
-
question_answer195) If you are asked to classify the various algae into distinct groups, which of the following characters you should choose?
A)
Types of pigments present in die cell
done
clear
B)
Nature of stored food materials in die cell
done
clear
C)
Structural organisation of thallus
done
clear
D)
Chemical composition of die cell wall
done
clear
View Answer play_arrow
-
question_answer196) A plant requires magnesium for:
A)
holding cells together
done
clear
B)
protein synthesis
done
clear
C)
chlorophyll synthesis
done
clear
D)
cell wall development
done
clear
View Answer play_arrow
-
question_answer197) The overall goal of glycolysis, Krebs cycle and the electron transport system is the formation of:
A)
ATP in small stepwise units
done
clear
B)
ATP in one large oxidation reaction
done
clear
C)
Sugars
done
clear
D)
Nucleic acids
done
clear
View Answer play_arrow
-
question_answer198) A high density of elephant population in an area can result in:
A)
Mutualism
done
clear
B)
lntraspecific competition
done
clear
C)
Interspecific competition
done
clear
D)
Predation on one another
done
clear
View Answer play_arrow
-
question_answer199) A drop of each of the following, is placed separately on four slides. Which of them will not coagulate?
A)
Blood plasma
done
clear
B)
Blood serum
done
clear
C)
Sample from die thoracic duct of lymphatic system
done
clear
D)
Whole blood from pulmonary vein
done
clear
View Answer play_arrow
-
question_answer200) For a critical study of secondary growth in plants, which one of the following pairs is suitable:
A)
Sugarcane and sunflower
done
clear
B)
Teak and pine
done
clear
C)
Deodar and fem
done
clear
D)
Wheat and maiden hair fern
done
clear
View Answer play_arrow
 How fast is the light travelling in the liquid? \[1.8\times {{10}^{8}}\,m/s\]
How fast is the light travelling in the liquid? \[1.8\times {{10}^{8}}\,m/s\] 
 The work done in charging fully both the condensers is:
The work done in charging fully both the condensers is:




