
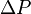 is the hydrostatic pressure (given in pascals in the SI system), or the difference in pressure at two points within a fluid column, due to the weight of the fluid;
ρ is the fluid density (in kilograms per cubic meter more...
is the hydrostatic pressure (given in pascals in the SI system), or the difference in pressure at two points within a fluid column, due to the weight of the fluid;
ρ is the fluid density (in kilograms per cubic meter more...
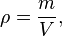 where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,although this is scientifically inaccurate – this quantity is more properly called specific weight.
Visit http://www.studyadda.com/videos/ix-class-physics-lectures/floatation/density/1744
All the video lectures at StudyAdda are exclusively developed and taught by Mr. Lalit Sardana(IIT-JEE,AIR 243) and Mrs. Shweta Sardana(AIPMT Trainer, MSc., MPhil (Gold Medalist)), Sardana Tutorials).
where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,although this is scientifically inaccurate – this quantity is more properly called specific weight.
Visit http://www.studyadda.com/videos/ix-class-physics-lectures/floatation/density/1744
All the video lectures at StudyAdda are exclusively developed and taught by Mr. Lalit Sardana(IIT-JEE,AIR 243) and Mrs. Shweta Sardana(AIPMT Trainer, MSc., MPhil (Gold Medalist)), Sardana Tutorials).
 
This principle has a variety of names, depending upon the discipline using it. See, for example, homeostasis. It is common to take Le Chatelier's principle to be a more general observation, roughly stated:
Any change in status quo prompts an opposing reaction in the responding system.
In chemistry, the principle is used to manipulate the outcomes of reversible reactions, often to increase the yield of reactions. In pharmacology, the binding of ligands to the receptor may shift more...

This principle has a variety of names, depending upon the discipline using it. See, for example, homeostasis. It is common to take Le Chatelier's principle to be a more general observation, roughly stated:
Any change in status quo prompts an opposing reaction in the responding system.
In chemistry, the principle is used to manipulate the outcomes of reversible reactions, often to increase the yield of reactions. In pharmacology, the binding of ligands to the receptor may shift more...
 
the thermodynamic equilibrium constant can be defined such that, at equilibrium,

the thermodynamic equilibrium constant can be defined such that, at equilibrium,
 
where curly brackets denote the thermodynamic activities of the chemical species. The logarithm of this expression appears in the formula for the Gibbs free energy change for the reaction. If deviations from ideal behaviour are neglected, the activities may be replaced by concentrations, [A], and a concentration quotient, Kc.

where curly brackets denote the thermodynamic activities of the chemical species. The logarithm of this expression appears in the formula for the Gibbs free energy change for the reaction. If deviations from ideal behaviour are neglected, the activities may be replaced by concentrations, [A], and a concentration quotient, Kc.
![K_text{c}=frac{{[R]} ^rho {[S]}^sigma ... } {{[A]}^alpha {[B]}^beta ...}](http://upload.wikimedia.org/math/3/9/5/3955cdeb8e83126bb0daea63876c7789.png) 
Kc is defined which is equal to the thermodynamic equilibrium constant divided by a quotient of activity coefficients. For ideal behaviour this quotient has a value of 1, and Kc = K

Kc is defined which is equal to the thermodynamic equilibrium constant divided by a quotient of activity coefficients. For ideal behaviour this quotient has a value of 1, and Kc = KYou need to login to perform this action.
You will be redirected in
3 sec
