question_answer 1) Which of the following has the highest number of significant figures?
A)
\[0.00\text{7}{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[\text{2}.\text{64}\times \text{l}{{0}^{\text{24}}}\text{kg}\]
done
clear
C)
\[0.000\text{6}0\text{32}{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{6}.\text{32}00\text{ J}\]
done
clear
View Answer play_arrow
question_answer 2)
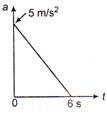
A particle starts from rest. Its acceleration at time \[\text{t}=0\text{ is 5 m}/{{\text{s}}^{\text{2}}}\]which varies with time as shown in the figure. The maximum speed of the particle will be
A)
7.5 m/s
done
clear
B)
15 m/s
done
clear
C)
30 m/s
done
clear
D)
37.5 m/s
done
clear
View Answer play_arrow
question_answer 3) A ball of mass 0.3 kg is tied to one end of a string 0.8 m long and rotated in a vertical circle. If the tension in the string be zero at the highest point of the circle, the tension at the lowest point in this situation will be equal to
A)
zero
done
clear
B)
2.94 N
done
clear
C)
17.6 N
done
clear
D)
19.60 N
done
clear
View Answer play_arrow
question_answer 4) If vectors A and B are such that |A+B|=|A|=|B|, then |A-B| may be equated to
A)
\[\frac{\sqrt{3}}{2}\left| \text{A} \right|\]
done
clear
B)
|A|
done
clear
C)
\[\frac{\sqrt{2}}{2}\left| \text{A} \right|\]
done
clear
D)
\[\sqrt{3}\]|A|
done
clear
View Answer play_arrow
question_answer 5) A particle of mass m moving with speed v towards east strikes another particle of same mass moving with same speed v towards north. After striking, the two particles fuse together. With what speed this new particle of mass 2m will move in north-east direction?
A)
\[v\]
done
clear
B)
\[\frac{v}{2}\]
done
clear
C)
\[\frac{v}{\sqrt{2}}\]
done
clear
D)
\[v\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 6)
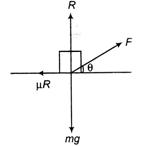
A body of mass m rests on a horizontal floor with which it has a coefficient of static friction 4. It is desired to make the body move by applying a minimum possible force F as shown in the diagram. The values of \[\theta \] and \[{{F}_{\min }}\]shall be respectively equal to
A)
\[{{\tan }^{-1}}\mu \frac{\mu mg}{\sqrt{(1+{{\mu }^{2}})}}\]
done
clear
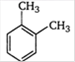
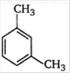
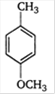
B)
\[{{\tan }^{-1}}\mu ,\frac{mg}{\sqrt{(1+{{\mu }^{2}})}}\]
done
clear
C)
\[{{\tan }^{-1}}\mu ,\frac{\mu mg}{\sqrt{(1-{{\mu }^{2}})}}\]
done
clear
D)
\[{{\tan }^{-1}}\mu ,\frac{mg}{\sqrt{(1-{{\mu }^{2}})}}\]
done
clear
View Answer play_arrow
question_answer 7)
Three blocks of masses m, 3m and 5m are connected by massless strings and pulled by a force F on a frictionless surface as shown in the figure below. The tension P in the first string is 16 N
A)
16 N, 10 N
done
clear
B)
10 N, 16 N
done
clear
C)
8 N, 2 N
done
clear
D)
10 N, 6 N
done
clear
View Answer play_arrow
question_answer 8) A particle is moved from a position \[{{r}_{1}}-\text{i}=\left( \text{3}\overset{\hat{\ }}{\mathop{\text{i}}}\,\text{ }+\text{ 2}\overset{\hat{\ }}{\mathop{\text{j}}}\,-\text{6k} \right)\]metre to a position \[{{r}_{2}}(14\overset{\hat{\ }}{\mathop{i}}\,+13\overset{\hat{\ }}{\mathop{j}}\,+9\overset{\hat{\ }}{\mathop{k}}\,)\]metre under the action of a force \[\text{F}=\left( \text{4}\overset{\hat{\ }}{\mathop{\text{i}}}\,+\overset{\hat{\ }}{\mathop{\text{j}}}\,+\text{3}\overset{\hat{\ }}{\mathop{\text{k}}}\, \right)\text{N}.\]The work done is equal to
A)
32 J
done
clear
B)
64 J
done
clear
C)
96 J
done
clear
D)
100 J
done
clear
View Answer play_arrow
question_answer 9) A body of mass 1.0 kg is rotating on a circular path of diameter 2.0 m at the rate of 10 rotations in 31.4 s. The angular momentum of the body, in \[kg-{{m}^{2}},s\]is
A)
1.0
done
clear
B)
1.5
done
clear
C)
2.0
done
clear
D)
4.0
done
clear
View Answer play_arrow
question_answer 10)
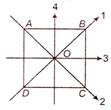
The moment of inertia of a thin square plate ABCD (shown in figure below) of uniform thickness about an axis passing through the centre 0 and perpendicular to the plane of the plate is
A)
\[{{I}_{1}}-{{I}_{2}}\]
done
clear
B)
\[{{I}_{1}}+{{I}_{2}}+{{I}_{3}}+{{I}_{4}}\]
done
clear
C)
\[{{I}_{1}}+{{I}_{3}}\]
done
clear
D)
\[{{I}_{1}}+{{I}_{4}}\]
done
clear
View Answer play_arrow
question_answer 11) A uniform rod of length 1.0 m is bent at: its mid-point to make \[90{}^\circ \] angle. The distance of the centre of mass from the centre of the rod is
A)
36.1 cm
done
clear
B)
25.2 cm
done
clear
C)
17.7 cm
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 12) A small planet is revolving around a very massive star in a circular orbit of radius R with a period of revolution T. If the gravitational force between the planet and the star were proportional to \[{{R}^{-5/2}},\] then T would be proportional to
A)
\[{{R}^{3/2}}\]
done
clear
B)
\[{{R}^{3/5}}\]
done
clear
C)
\[{{R}^{7/2}}\]
done
clear
D)
\[{{R}^{7/4}}\]
done
clear
View Answer play_arrow
question_answer 13) An artificial satellite moving in a circular orbit around the earth has a total (kinetic + potential) energy eq. Its potential energy is
A)
\[-{{E}_{o}}\]
done
clear
B)
\[{{E}_{o}}\]
done
clear
C)
\[-2{{E}_{o}}\]
done
clear
D)
\[2{{E}_{o}}\]
done
clear
View Answer play_arrow
question_answer 14) 4 J of work is required to stretch a spring through 10 cm beyond its unstretched length. The extra work required to stretch it through additional 10 cm shall be
A)
4 J
done
clear
B)
8 J
done
clear
C)
12 J
done
clear
D)
16 J
done
clear
View Answer play_arrow
question_answer 15) Consider a soap film on a rectangular frame of wire of area \[\text{4}\times \text{4 cm2}.\] If the area of the soap film is increased to \[\text{4}\times 5\text{ cm2,}\] the work done in the process will be
A)
\[12\times {{10}^{-6}}J\]
done
clear
B)
\[24\times {{10}^{-6}}J\]
done
clear
C)
\[60\times {{10}^{-6}}J\]
done
clear
D)
\[96\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 16) (The surface tension of the soap film is \[\text{3}\times \text{1}{{0}^{-\text{2}}}\text{ N}/\text{m}).\] A drop of water of radius 0.0015 mm is falling in air. If the coefficient of viscosity of air is \[\text{2}.0\times \text{1}{{0}^{-\text{5}}}\text{ kg}/\text{ms},\] the terminal velocity of the drop will be
A)
\[\text{1}.0\times \text{1}{{0}^{-\text{4}}}\text{ m}/\text{s}\]
done
clear
B)
\[2.0\times \text{1}{{0}^{-\text{4}}}\text{ m}/\text{s}\]
done
clear
C)
\[2.5\times \text{1}{{0}^{-\text{4}}}\text{ m}/\text{s}\]
done
clear
D)
\[5.0\times \text{1}{{0}^{-\text{4}}}\text{ m}/\text{s}\]
done
clear
View Answer play_arrow
question_answer 17)
The potential energy U of two atoms of a diatomic molecule as a function of distance r between the atoms is shown in the given figure.
A)
1 only
done
clear
B)
2 only
done
clear
C)
3 only
done
clear
D)
2 and 3
done
clear
View Answer play_arrow
question_answer 18)
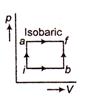
In the given figure, the initial and final states of a gas are shown by points \[i\]and \[f.\] The internal energy of the gas at i is 10 J. For the path ibf; dQ = 50 J and dW = 20 J. For the path ibf, if dQ = 36 J the value of dW will be equal to
A)
4 J
done
clear
B)
12 J
done
clear
C)
18 J
done
clear
D)
6 J
done
clear
View Answer play_arrow
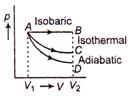
question_answer 19)
A gas is expanded from volume V1 to volume \[{{V}_{2}}\]in three processes, shown in the figure. If \[{{U}_{A}},\]\[{{U}_{B}},\]\[{{U}_{C}}\] and \[{{U}_{D}}\]represent the internal energies of the gas when in state A, B, C and D respectively, then which of the following is not correct?
A)
\[{{U}_{B}}-{{U}_{A}}=0\]
done
clear
B)
\[{{U}_{C}}-{{U}_{A}}=0\]
done
clear
C)
\[{{U}_{D}}-{{U}_{A}}<0\]
done
clear
D)
\[{{U}_{B}}-{{U}_{C}}<0\]
done
clear
View Answer play_arrow
question_answer 20) The average separation between the molecules of 1 L of an ideal gas at NTP may be equated to
A)
\[3.3\times {{10}^{-10}}m\]
done
clear
B)
\[3.3\times {{10}^{-9}}m\]
done
clear
C)
\[3.3\times {{10}^{-8}}m\]
done
clear
D)
\[3.3\times {{10}^{-7}}m\]
done
clear
View Answer play_arrow
question_answer 21) During an experiment, an ideal gas is found to obey an additional law \[\text{V}{{\text{p}}^{\text{2}}}=\]constant. The gas is initially at temperature T and volume V. When it expands to a volume 2V, the temperature becomes
A)
T
done
clear
B)
2 T
done
clear
C)
3 T
done
clear
D)
T/2
done
clear
View Answer play_arrow
question_answer 22) A person measures the time period of a simple pendulum inside a stationary lift and finds it to be T. If the lift starts accelerating upwards with an acceleration g/3, the time period of the pendulum will be
A)
\[\sqrt{3}T\]
done
clear
B)
\[T/\sqrt{3}\]
done
clear
C)
\[\frac{\sqrt{3}T}{2}\]
done
clear
D)
T/3
done
clear
View Answer play_arrow
question_answer 23) The relation between time and displacement for two particles is given by (i) \[{{y}_{1}}=0.03\sin \,2\pi t(60t+{{\phi }_{1}})m\] (ii) \[{{y}_{2}}=0.06\sin \,2\pi t(60t+{{\phi }_{2}})m\] The ratio of intensities \[{{I}_{1}}:{{I}_{2}}\]of the two waves generated by the vibrations of the two particles in a given medium will be
A)
1 :1
done
clear
B)
1 : 2
done
clear
C)
1 : 4
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 24) The transverse displacement of a string fixed at both ends is given by \[y=0.06\sin \left( \frac{2\pi x}{3} \right)\cos (100\pi t)\]where \[x\]and \[y\] are in metre and t is in second. The length of the string is 1.5 m and its mass is \[\text{3}.0\times \text{1}{{0}^{-\text{2}}}\text{ kg}.\]What is die tension in the string?
A)
225 N
done
clear
B)
300 N
done
clear
C)
450 N
done
clear
D)
675 N
done
clear
View Answer play_arrow
question_answer 25) An observer moves towards a stationary source of sound with a velocity one - tenth the velocity of sound. The apparent increase in frequency is
A)
zero
done
clear
B)
2.5%
done
clear
C)
5%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 26) A spherical conducting shell of inner radius \[{{r}_{1}}\]and outer radius \[{{r}_{2}}\]has a charge Q. A charge -q is placed at the centre of the shell. The surface charge density on the inner and outer surfaces of the shell will be
A)
\[\frac{q}{4\pi r_{2}^{1}}and\frac{Q}{4\pi r_{2}^{2}}\]
done
clear
B)
\[\frac{-q}{4\pi r_{2}^{1}}and\frac{Q+q}{4\pi r_{2}^{2}}\]
done
clear
C)
\[\frac{q}{4\pi r_{1}^{2}}and\frac{Q-q}{4\pi r_{2}^{2}}\]
done
clear
D)
\[0\,and\,\frac{Q-q}{4\pi r_{2}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) A 600 pF capacitor is charged by 200 V supply. It is then disconnected from the supply and is connected to another 300 pF capacitor. The electrostatic energy lost in the process is
A)
zero
done
clear
B)
\[4\times {{10}^{-6}}J\]
done
clear
C)
\[6\times {{10}^{-6}}J\]
done
clear
D)
\[8\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 28) In a field free region, two electrons are released to move on a line towards each other with velocities 106 m/s. The distance of their closest approach will be nearer to
A)
\[1.28\times {{10}^{-10}}m\]
done
clear
B)
\[1.92\times {{10}^{10}}m\]
done
clear
C)
\[2.56\times {{10}^{-10}}m\]
done
clear
D)
\[3.84\times {{10}^{10}}m\]
done
clear
View Answer play_arrow
question_answer 29)
Two identical capacitors \[{{C}_{1}}\]and \[{{C}_{2}}\]are connected as shown in the figure with a battery B. A dielectric slab is slipped between the plates of the capacitor \[{{C}_{2}},\]the battery remaining connected. Consider the following changes in the charges on the capacitors and the potential difference across their plates
A)
(i), (ii), (iii) and (iv)
done
clear
B)
(ii) only
done
clear
C)
(ii) and (iv)
done
clear
D)
(i) and (iv)
done
clear
View Answer play_arrow
question_answer 30) A charged drop of mass \[\text{3}.\text{2}\times \text{1}{{0}^{-\text{12}}}\text{ g}\]floats between two horizontal parallel plates maintained at potential difference of 980 V and separation between the plates 2 cm. The number of excess or deficient electrons on the drop is
A)
2
done
clear
B)
4
done
clear
C)
8
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 31) The number density of free electrons in a copper conductor is \[8.5\times {{10}^{28}}.\,{{m}^{-3}}\] How long does an electron take to drift from one end of a wire 3.0 m long to its other end? The area of cross-section of the wire is \[2.0\times {{10}^{-6}}{{m}^{2}}\]and it is carrying a current of 3.0A.
A)
\[8.1\times {{10}^{4}}s\]
done
clear
B)
\[2.7\times {{10}^{4}}s\]
done
clear
C)
\[9\times {{10}^{3}}s\]
done
clear
D)
\[3\times {{10}^{3}}s\]
done
clear
View Answer play_arrow
question_answer 32) Two wires A and B of equal masses and of the same metal are taken. The diameter of the wire A is half the diameter of the wire B. If the resistance of wire A be 24\[\Omega \], the resistance of B will be
A)
3 \[\Omega \]
done
clear
B)
1.5 \[\Omega \]
done
clear
C)
4.5 \[\Omega \]
done
clear
D)
6.0 \[\Omega \]
done
clear
View Answer play_arrow
question_answer 33)
Figure shows three resistor configurations \[{{R}_{1}},{{R}_{2}}\]and \[{{R}_{3}}\]connected to 3 V battery.
A)
\[{{P}_{1}}>{{P}_{2}}>{{P}_{3}}\]
done
clear
B)
\[{{P}_{1}}>{{P}_{3}}>{{P}_{2}}\]
done
clear
C)
\[{{P}_{2}}>{{P}_{1}}>{{P}_{3}}\]
done
clear
D)
\[{{P}_{3}}>{{P}_{2}}>{{P}_{1}}\]
done
clear
View Answer play_arrow
question_answer 34) An electron having momentum \[2.4\times {{10}^{-23}}\text{ }kg-m/s\]enters a region of uniform magnetic field of 0.15 T. The field vector makes an angle of 30° with the initial velocity vector of the electron. The radius of the helical path of the electron in the field shall be
A)
\[2\,m\,m\]
done
clear
B)
\[1\,m\,m\]
done
clear
C)
\[\frac{\sqrt{3}}{2}m\,m\]
done
clear
D)
\[0.5\,m\,m\]
done
clear
View Answer play_arrow
question_answer 35) A closely wound solenoid 80 cm long has 5 layers of winding of 400 turns each. The diameter of the solenoid is 1.8 cm. If the current carried is 8.0 A, the magnitude of B inside the solenoid near its centre will be
A)
\[8\pi \times {{10}^{-3}}T\]
done
clear
B)
\[6\pi \times {{10}^{-3}}T\]
done
clear
C)
\[p-n\]
done
clear
D)
\[3\pi \times {{10}^{-3}}T\]
done
clear
View Answer play_arrow
question_answer 36) A circular coil of 20 turns and radius 10 cm is placed in a uniform magnetic field of 0.10 T normal to the plane of the coil. If the current in the coil is 5.0 A, the total torque on the coil will be
A)
zero
done
clear
B)
0.314 J
done
clear
C)
3.14 J
done
clear
D)
6.28 J
done
clear
View Answer play_arrow
question_answer 37) A 1.0 m long metallic rod is rotated with an angular frequency of 400 rad /s about an axis normal to the rod passing through its one end. A constant and uniform magnetic field of 0.5 T parallel to the axis exists everywhere. The emf developed across the ends of the rod is
A)
50 V
done
clear
B)
100 V
done
clear
C)
150 V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) Materials suitable for permanent magnets, must have which of the following properties?
A)
High retentivity, low coercivity and high permeability
done
clear
B)
Low retentivity, low coercivity and low permeability
done
clear
C)
Low retentivity, high coercivity and low permeability
done
clear
D)
High retentivity, high coercivity and high permeability
done
clear
View Answer play_arrow
question_answer 39) An alternating voltage (in volt) given by \[V=200\sqrt{2}\]sin(100\[t\]) is connected to \[1\mu F\] capacitor through an ideal AC ammeter in series. The reading of the ammeter and the average power consumed in the circuit shall be
A)
\[20\text{ }mA,\text{ }0\]
done
clear
B)
\[20mA,4W\]
done
clear
C)
\[20\sqrt{2}\,mA,8W\]
done
clear
D)
\[20\sqrt{2}\,mA,4\sqrt{2}W\]
done
clear
View Answer play_arrow
question_answer 40)
The figure shows a L-C-R network connected to 300 V AC supply. The circuit elements are such that \[R={{X}_{L}}={{X}_{C}}=10\Omega .\,{{V}_{1}},{{V}_{2}}\]and \[{{V}_{3}}\]are three AC voltmeters connected as shown in the figure. Which of the following represents the correct set of readings of the voltmeters?
A)
\[{{V}_{1}}=100\text{ }V,{{V}_{2}}=100\text{ }V,{{V}_{3}}=100\text{ }V\]
done
clear
B)
\[{{V}_{1}}\text{ }=\text{ }150V,{{V}_{2}}\text{ }=\text{ }zero,\text{ }{{V}_{3}}\text{ }=\text{ }150\text{ }V\]
done
clear
C)
\[{{V}_{1}}=300\text{ }V,{{V}_{2}}=100\text{ }V,{{V}_{3}}=100\text{ }V\]
done
clear
D)
\[{{V}_{1}}=300\text{ }V,\text{ }{{V}_{2}}=300V,{{V}_{3}}=300\text{ }V\]
done
clear
View Answer play_arrow
question_answer 41) A plane electromagnetic wave of frequency 25 MHz travels in free space along the x-direction. At a particular point in space and time, E = 6.3j V/m. At this point B is equal to
A)
\[8.33\times {{10}^{-8}}\overset{\hat{\ }}{\mathop{k}}\,T\]
done
clear
B)
\[18.9\times {{10}^{-8}}\overset{\hat{\ }}{\mathop{k}}\,T\]
done
clear
C)
\[2.1\times {{10}^{-8}}\overset{\hat{\ }}{\mathop{k}}\,T\]
done
clear
D)
\[2.1\times {{10}^{8}}\overset{\hat{\ }}{\mathop{k}}\,T\]
done
clear
View Answer play_arrow
question_answer 42)
If the behaviour of light rays is as shown in the figure. The relation between refractive indices\[\mu \],\[{{\mu }_{1}}\].i and \[{{\mu }_{2}}\]is
A)
\[\mu >{{\mu }_{2}}>{{\mu }_{1}}\]
done
clear
B)
\[\mu <{{\mu }_{2}}<{{\mu }_{1}}\]
done
clear
C)
\[\mu <{{\mu }_{2}},\mu ={{\mu }_{1}}\]
done
clear
D)
\[{{\mu }_{2}}<{{\mu }_{1}};\mu ={{\mu }_{2}}\]
done
clear
View Answer play_arrow
question_answer 43) When a telescope is in normal adjustment, the distance of the objective from the eyepiece is found to be 100 cm. If the magnifying power of the telescope, at normal adjustment, is 24, the focal lengths of the lenses are
A)
96 cm, 4 cm
done
clear
B)
48 cm, 2 cm
done
clear
C)
50 cm, 50 cm
done
clear
D)
80 cm, 20 cm
done
clear
View Answer play_arrow
question_answer 44) In Youngs double-slit experiment the distance between the centres of adjacent fringes is 0.10 mm. If the distance of the screen from the slits is doubled, the distance between the slits is halved and the wavelength of light is changed from \[6.4\times {{10}^{-7}}\text{ }m\text{ }to\text{ }4.0\times {{10}^{-7}}m,\] then the new distance between the fringes will be
A)
0.10 mm
done
clear
B)
0.15 mm
done
clear
C)
0.20 mm
done
clear
D)
0.25 mm
done
clear
View Answer play_arrow
question_answer 45) A transparent thin plate of a polaroid is placed on another similar plate such that the angle between their axes is 30°. The intensities of the emergent and the unpolarised incident light will be in the ratio of
A)
1 : 4
done
clear
B)
1 : 3
done
clear
C)
3 : 4
done
clear
D)
3 : 8
done
clear
View Answer play_arrow
question_answer 46) Work function of potassium metal is 2.30 eV. When light of frequency \[8\times 1014\text{ }Hz\]is incident on the metal surface, photoemission of electrons occurs. The stopping potential of the electrons will be equal to
A)
0.1 V
done
clear
B)
1.0 V
done
clear
C)
5.0 V
done
clear
D)
3.3 V
done
clear
View Answer play_arrow
question_answer 47) The radioactive decay constant of \[_{38}^{90}Sr\]is\[7.88\times {{10}^{-10}}{{s}^{-1}}.\]The activity of 15 mg of this isotope will be
A)
1.5 Ci
done
clear
B)
2.13 Ci
done
clear
C)
7.88 Ci
done
clear
D)
8.76 Ci
done
clear
View Answer play_arrow
question_answer 48) Consider the following reaction \[_{1}^{1}H+_{1}^{3}H+_{1}^{2}H\] The atomic masses are given as \[m(_{1}^{1}H)=1.007825\,u\] \[m(_{1}^{2}H)=2.014104\,u\] \[m(_{1}^{3}H)=3.016049\,u\] The Q-value of the above reaction will be
A)
- 4.03 MeV
done
clear
B)
-2.01 MeV
done
clear
C)
2.01 MeV
done
clear
D)
4.03 MeV
done
clear
View Answer play_arrow
question_answer 49)
The circuit shown in the figure contains three diodes each with forward resistance of 50\[\Omega \] and with infinite backward resistance. If the battery voltage is 6 V, the current through the 100 \[\Omega \] resistance is
A)
zero
done
clear
B)
36 mA
done
clear
C)
43 mA
done
clear
D)
50 mA
done
clear
View Answer play_arrow
question_answer 50)
Two identical p-n junctions are connected in series with a battery in three ways, as shown below
A)
Circuit 1 and Circuit 2
done
clear
B)
Circuit 2 and Circuit 3
done
clear
C)
Circuit 3 and Circuit 1
done
clear
D)
Circuit 2 only
done
clear
View Answer play_arrow
question_answer 51) Example of electrophile is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[S{{O}_{3}}\]
done
clear
D)
\[RN{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 52) Which one of the following is the most electronegative element configuration?
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}\]
done
clear
D)
\[I{{s}^{2}},\text{ }2{{s}^{2}}\text{ }2{{p}^{5}}\]
done
clear
View Answer play_arrow
question_answer 53) Splitting of spectral lines in electric field is called
A)
Zeeman effect
done
clear
B)
Stark effect
done
clear
C)
Photoelectric effect
done
clear
D)
Shielding effect
done
clear
View Answer play_arrow
question_answer 54) The energy of electron in the first orbit of hydrogen atom is
A)
\[-2.18\times {{10}^{-18}}\text{ }J\text{ }ato{{m}^{-1}}\]
done
clear
B)
\[-2.18\times {{10}^{-18}}\text{ k}J\text{ }ato{{m}^{-1}}\]
done
clear
C)
\[-2.18\times {{10}^{-18}}\text{ ergs }ato{{m}^{-1}}\]
done
clear
D)
\[+2.18\times {{10}^{-18}}\text{ J }ato{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 55) Solid \[C{{O}_{2}}\] is an example of
A)
covalent solid
done
clear
B)
metallic solid
done
clear
C)
molecular solid
done
clear
D)
ionic solid
done
clear
View Answer play_arrow
question_answer 56) \[S{{F}_{4}}\] can be classified as
A)
\[A{{B}_{4}}\] type
done
clear
B)
\[A{{B}_{4}}\] E type
done
clear
C)
\[A{{B}_{4}}{{E}_{2}}\] type
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 57) For the reaction, \[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\] equilibrium constant can be given
A)
\[{{K}_{p}}{{K}_{c}}{{[RT]}^{-2}}\]
done
clear
B)
\[{{K}_{p}}{{K}_{c}}{{[RT]}^{2}}\]
done
clear
C)
\[{{K}_{p}}{{K}_{c}}{{[RT]}^{{}}}\]
done
clear
D)
\[{{K}_{p}}{{K}_{c}}{{[nRT]}^{{}}}\]
done
clear
View Answer play_arrow
question_answer 58) Heating of \[N{{H}_{4}}N{{O}_{3}}\] gives
A)
\[{{N}_{2}}O\] gas
done
clear
B)
NO gas
done
clear
C)
\[N{{O}_{2}}\] gas
done
clear
D)
\[{{O}_{2}}\] gas
done
clear
View Answer play_arrow
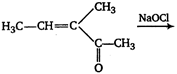
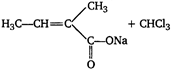
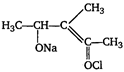
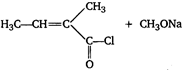
question_answer 59)
In the following reaction,
A)
\[N{{H}_{2}}\]
done
clear
B)
NHOH
done
clear
C)
\[{{N}^{+}}{{H}_{3}}C{{l}^{-}}\]
done
clear
D)
\[N=N-{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 60)
In the reaction
A)
ethylamine, ethanol
done
clear
B)
ethylamine, sodium cyanoborohydride
done
clear
C)
ethylamine, hydrogen peroxide
done
clear
D)
ethylalcohol, sodium metal
done
clear
View Answer play_arrow
question_answer 61) Decreasing order of stability of hydrogen halide is
A)
HF > HCl > HBr > HI
done
clear
B)
HF < HCl < HBr < HI
done
clear
C)
HP > HCl < HBr = HI
done
clear
D)
HF > HCl = HBr > HI
done
clear
View Answer play_arrow
question_answer 62) Which one of the following has maximum lattice energy?
A)
LiF
done
clear
B)
LiCI
done
clear
C)
UBr
done
clear
D)
Lil
done
clear
View Answer play_arrow
question_answer 63) Oxidising species in the following reaction is\[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Z{{n}^{2+}}(aq)+cu\]
A)
Zn
done
clear
B)
\[C{{u}^{2+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 64) The rate constant of a reaction is \[mol\text{ }{{L}^{-}}{{S}^{-1}}.\] The reaction is
A)
first order
done
clear
B)
second order
done
clear
C)
zero order
done
clear
D)
pseudo first order
done
clear
View Answer play_arrow
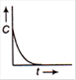
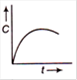
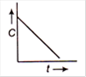
question_answer 65) The plot of concentration of the reactant versus time for a zero order reaction is given By
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 66) Colloidal particles carry
A)
electrical charge
done
clear
B)
no electrical charge
done
clear
C)
same charge in a given colloidal solution
done
clear
D)
different charges in a given colloidal solution
done
clear
View Answer play_arrow
question_answer 67) \[{{E}^{o}}\] cell of a galvanic cell is 1.1 V. Standard free energy change can be given as
A)
- 212.27 kJ \[mo{{l}^{-1}}\]
done
clear
B)
- 212.27 J \[mo{{l}^{-1}}\]
done
clear
C)
+ 212.27kJ \[mo{{l}^{-1}}\]
done
clear
D)
+ 212.27V
done
clear
View Answer play_arrow
question_answer 68) The standard potentials at room temperature for the two half cells are given \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn,\,\,{{E}^{o}}=-0.762\,V\] \[M{{g}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Mg,\text{ }\,\,E{}^\circ =-\text{ }2.37\,V\] When Zn metal is added to a solution of \[MgC{{l}_{2}}\]
A)
\[ZnC{{l}_{2}}\] is formed
done
clear
B)
Mg is precipitated
done
clear
C)
Zn dissolves in the solution
done
clear
D)
no reaction takes place
done
clear
View Answer play_arrow
question_answer 69) For the reaction, \[CO+(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
A)
\[\Delta \,H\,=\,\,\Delta \,E\]
done
clear
B)
\[\Delta \,H\,>\,\,\Delta \,E\]
done
clear
C)
\[\Delta \,H\,\,<\,\,\Delta \,E\]
done
clear
D)
\[\Delta \,H\,\,-\,\,\Delta \,E=0\]
done
clear
View Answer play_arrow
question_answer 70) The maximum precipitating power for negatively charged \[A{{s}_{2}}{{S}_{3}}\] sol is exhibited by
A)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[CaC{{l}_{2}}\]
done
clear
C)
\[N{{a}_{3}}P{{O}_{4}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 71) \[\Delta S\] is expected to be maximum for the reaction
A)
\[Ca\,(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}CaO\,(s)\]
done
clear
B)
\[C\,(g)+{{O}_{2}}(g)\xrightarrow{{}}{{O}_{2}}\left( g \right)\]
done
clear
C)
\[{{N}_{2}}\,(g)+{{O}_{2}}(g)\xrightarrow{{}}2N{{O}_{2}}(g)\]
done
clear
D)
\[CaC{{O}_{3}}\,(s)\xrightarrow{{}}CaO\,(s)+C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following is an example of Maximum boiling azeotrope?
A)
2 % KCl solution in water
done
clear
B)
90 % ethanol + 10% water
done
clear
C)
68 % \[HN{{O}_{3}}+32%\] water
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 73) Which of the following is not affected by temperature change?
A)
Molarity
done
clear
B)
Normality
done
clear
C)
Molality
done
clear
D)
Formality
done
clear
View Answer play_arrow
question_answer 74) The oxide appears blue in solid states in
A)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 75) The noble gas not found in the atmosphere is
A)
He
done
clear
B)
Kr
done
clear
C)
As
done
clear
D)
Rn
done
clear
View Answer play_arrow
question_answer 76) n-type of semiconductor can be prepared by doping silicon with
A)
Ni
done
clear
B)
Zn
done
clear
C)
As
done
clear
D)
Al
done
clear
View Answer play_arrow
question_answer 77) The gas equation pV = nZRT becomes ideal gas equation when
A)
Z = 0
done
clear
B)
Z = 0.5
done
clear
C)
Z = 1
done
clear
D)
Z = 2
done
clear
View Answer play_arrow
question_answer 78) The interaction between a polar and non-polar molecule is known as
A)
dipole-dipole interaction
done
clear
B)
dipole-induced dipole interaction
done
clear
C)
ion-dipole interaction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 79) Which of the following releases maximum energy on combustion in kJ per mole state?
A)
Dihydrogen in gaseous state
done
clear
B)
LPG
done
clear
C)
\[C{{H}_{4}}\] gas
done
clear
D)
Octane in liquid state
done
clear
View Answer play_arrow
question_answer 80) In diborane, each boron atom uses
A)
\[s{{p}^{2}}\]-hybrid orbitals for bonding
done
clear
B)
p-orbitals for bonding
done
clear
C)
\[s{{p}^{3}}\]-hybrid orbitals for bonding
done
clear
D)
\[ds{{p}^{2}}\] hybrid for bonding
done
clear
View Answer play_arrow
question_answer 81) \[T{{i}^{3+}}\]transition metal ion is purple in colour while \[T{{i}^{4+}}\] metal ion is colourless due to
A)
vacant d-orbitals
done
clear
B)
unpaired electrons in d orbitals
done
clear
C)
completely filled d-orbitals
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) Potassium dichromate in acidic solution reacts with \[F{{e}^{2+}}\]gives
A)
\[C{{r}^{3+}}+n{{H}_{2}}O\]
done
clear
B)
\[C{{r}^{+}}+n{{H}_{2}}O\]
done
clear
C)
\[C{{r}^{2+}}+n{{H}_{2}}O\]
done
clear
D)
\[C{{r}^{0}}+n{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 83) Which one of the following is heteroleptic complex?
A)
\[{{[CO{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[CO{{(CN)}_{6}}]}^{3}}\]
done
clear
C)
\[{{[CO{{(NH{{ }_{3}})}_{4}}C{{I}_{2}}]}^{+}}\]
done
clear
D)
\[{{[CO{{({{H}_{2}}O )}_{6}}]}^{+}}\]
done
clear
View Answer play_arrow
question_answer 84) Weak field ligands form high spin complexes due to (where \[{{\Delta }_{o}}\] is crystal field splitting and P = pairing energy)
A)
\[{{\Delta }_{0}}>P\]
done
clear
B)
\[{{\Delta }_{0}}<P\]
done
clear
C)
\[{{\Delta }_{0}}=P\]
done
clear
D)
\[{{\Delta }_{0}}=0\]
done
clear
View Answer play_arrow
question_answer 85) Which metal is used as phosphorescence
A)
W
done
clear
B)
Tb
done
clear
C)
Ac
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 86)
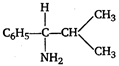
The IUPAC name of the compound is
A)
l-amino-2-methyl-l-phenyl propane
done
clear
B)
l-amino-l-phenyl-2-methyl propane
done
clear
C)
2-methyl-l-amino-l-phenyl propane
done
clear
D)
1-isopropyl-l-phenyl methyl amine
done
clear
View Answer play_arrow
question_answer 87) Reactivity of acid derivatives towards acyl substitution reaction is
A)
\[RCOC1\text{ }<\text{ }(RC{{O}_{2}}\text{)O }<\text{ }RCOOR\text{ }<\text{ }RCON{{H}_{2}}\]
done
clear
B)
\[RCOC1\text{ }{{(RCO\text{)}}_{2}}\text{O }RCOOR\text{ }RCON{{H}_{2}}\]
done
clear
C)
\[RCOC1\text{ }>\text{ }RCOOR\text{ }>\text{ }{{(RCO)}_{2}}O\text{ }>\text{ }RCON{{H}_{2}}\]
done
clear
D)
\[RCOC1\text{ }>\text{ }RCOOR\text{ }>\text{ }(RCON{{H}_{2}}\text{ }>\text{ (}RCO{{)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 88) 2-butanamine was added to the solution of sodium nitrite in hydrochloric acid. Gas liberated in this reaction is
A)
\[{{N}_{3}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 89)
Product(s) of the following reaction is (are)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 90) Which of the following will not undergo disproportionation reaction on heating with concentrated alkali?
A)
Benzaldehyde
done
clear
B)
Methanal
done
clear
C)
2,2-dimethyl propanal
done
clear
D)
Propanal
done
clear
View Answer play_arrow
question_answer 91) Acetophenone is a common name of
A)
\[{{(C{{H}_{3}})}_{2}}CO\]
done
clear
B)
\[C{{H}_{3}}OC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) Which of the following ethers is unlikely to be cleaved by hot cone. HBr?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) Phenol is manufactured from hydrocarbon on air oxidation followed by acidic hydrolysis. Compound is
A)
t-butylbenzene
done
clear
B)
ethylbenzene
done
clear
C)
isopropylbenzene
done
clear
D)
methylbenzene
done
clear
View Answer play_arrow
question_answer 94) Which of the following reaction is not correct?
A)
Propene \[+HC1\text{ }\to \text{ }2-\] chloropropane
done
clear
B)
Propene \[+HBr\xrightarrow{peroxide}\]
done
clear
C)
1-bromopropane Propene \[+HCl\xrightarrow{peroxide}\] 2-chloropropane
done
clear
D)
Propene \[+HBr\to \]1-bromopropane
done
clear
View Answer play_arrow
question_answer 95) Propyne is produced during the hydrolysis of
A)
SiC
done
clear
B)
\[A{{l}_{4}}{{C}_{3}}\]
done
clear
C)
\[M{{g}_{2}}{{C}_{3}}\]
done
clear
D)
\[Ca{{C}_{2}}\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following dicarboxylic acids gives a cyclic anhydride on heating?
A)
\[HOO{{(C{{H}_{2}})}_{2}}COOH\]
done
clear
B)
\[C{{H}_{2}}{{\left( COOH \right)}_{2}}\]
done
clear
C)
\[HOOC{{\left( C{{H}_{2}} \right)}_{4}}COOH\]
done
clear
D)
\[HOOC{{\left( C{{H}_{2}} \right)}_{5}}COOH\]
done
clear
View Answer play_arrow
question_answer 97) Which one of the following is not a xylene?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 98) Which of the following amino acids do not have primary amino functional group?
A)
Alanine
done
clear
B)
Isoleucine
done
clear
C)
Leucine
done
clear
D)
Proline
done
clear
View Answer play_arrow
question_answer 99) Which of the following is used as tranquilizers?
A)
Ranitidine
done
clear
B)
Chloroxylenol
done
clear
C)
Ofloxacin
done
clear
D)
Veronal
done
clear
View Answer play_arrow
question_answer 100) Eutrophication is due to
A)
\[SO_{4}^{2-}\]ion present in water
done
clear
B)
NaCI present in water
done
clear
C)
\[PO_{4}^{3-}\] present in water
done
clear
D)
heavy metal present in water
done
clear
View Answer play_arrow
question_answer 101) Central dogna in biology includes
A)
transcription
done
clear
B)
translation
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 102) Homeostasis refers to
A)
positive feedback
done
clear
B)
negative feedback
done
clear
C)
Both (a) and (b)
done
clear
D)
it is not a feedback
done
clear
View Answer play_arrow
question_answer 103) The first organism to be found on a bare rock is a/an
A)
mass
done
clear
B)
alga
done
clear
C)
lichen
done
clear
D)
fern
done
clear
View Answer play_arrow
question_answer 104) Postnatal tail can be traced in
A)
cobra
done
clear
B)
earthworm
done
clear
C)
scorpion
done
clear
D)
centipede
done
clear
View Answer play_arrow
question_answer 105) Highest cranial capacity is present in
A)
Homo sapiens sapiens (modem man)
done
clear
B)
Cro - magnon man
done
clear
C)
Peking man
done
clear
D)
Neanderthal man
done
clear
View Answer play_arrow
question_answer 106) Mammalia is a
A)
category
done
clear
B)
taxon
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 107) Which of the following is a prokaryote?
A)
Chlorella
done
clear
B)
Chlamydomonas
done
clear
C)
Protomyces
done
clear
D)
Oscillatoria
done
clear
View Answer play_arrow
question_answer 108) Algae which are called gulf weed are
A)
Chlamydomonas
done
clear
B)
Volvox
done
clear
C)
Chara
done
clear
D)
Sargassum
done
clear
View Answer play_arrow
question_answer 109) Sleeping sickness is caused by
A)
Trypanosoma
done
clear
B)
Leishmania
done
clear
C)
Entamoeba
done
clear
D)
Plasmodium
done
clear
View Answer play_arrow
question_answer 110) Diplanerism is exhibited by
A)
Saproiegnia
done
clear
B)
Rhisopus and Mucor
done
clear
C)
Ulothrix
done
clear
D)
Vauchena
done
clear
View Answer play_arrow
question_answer 111) Which of these does not show heterospory?
A)
Pinus
done
clear
B)
Selaginella
done
clear
C)
Dryopieris and moss
done
clear
D)
Sunflower
done
clear
View Answer play_arrow
question_answer 112) Which of the following groups is a side Sine in the process of evolution?
A)
Porifera
done
clear
B)
Annelids
done
clear
C)
Echinodemata
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) Which one of the following lacks sinus venosus?
A)
Birds
done
clear
B)
Amphibians
done
clear
C)
Mammals
done
clear
D)
Fish
done
clear
View Answer play_arrow
question_answer 114) Which of the following have equal placement in the five kingdom classification on the basis of nutrition?
A)
Morera and Protista
done
clear
B)
Protista and Plantae
done
clear
C)
Fungi and Animalia
done
clear
D)
Plantae and Animalia
done
clear
View Answer play_arrow
question_answer 115) In eukaryoric cells, the amount of basic proteins is often found to be
A)
more than the amount of DNA
done
clear
B)
less than the amount of DNA
done
clear
C)
equal 10 the amount to DNA
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 116) Fluid mosaic model exhibits amphipathy because of
A)
glycoproceins
done
clear
B)
phospholipids
done
clear
C)
lipids
done
clear
D)
glycolipids
done
clear
View Answer play_arrow
question_answer 117) The component of cell wall of the vessel of an angiospenn is a complex compound of
A)
glucose
done
clear
B)
galacmronic acid
done
clear
C)
coniferyl alcohol
done
clear
D)
chitin
done
clear
View Answer play_arrow
question_answer 118) Protein packaging is done in
A)
Golgi apparatus
done
clear
B)
ribosomes
done
clear
C)
ER
done
clear
D)
nucleolus
done
clear
View Answer play_arrow
question_answer 119) Hypochronic anaemia is caused by deficiency of
A)
Hb
done
clear
B)
iron
done
clear
C)
vitamin\[\text{-}{{\text{B}}_{\text{12}}}~~~~~~\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 120) he fastest acting enzyme in the biological kingdom is
A)
ligase
done
clear
B)
amylase
done
clear
C)
carboxypepticiase
done
clear
D)
carbonic anhydrase
done
clear
View Answer play_arrow
question_answer 121) Spindle fibres are made up of
A)
actin with proteins and RNA
done
clear
B)
cellulose and RNA
done
clear
C)
tubulin protein and RNA
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 122) Inorganic prosthetic group is
A)
coenzyme
done
clear
B)
activator
done
clear
C)
hormone
done
clear
D)
apoenzyme
done
clear
View Answer play_arrow
question_answer 123) 1 : 2 : 1 phenotypic and genoiypic ratio is found is
A)
complementary genes
done
clear
B)
blending inheritance
done
clear
C)
multiple alleles
done
clear
D)
pseudoalleles
done
clear
View Answer play_arrow
question_answer 124) What is the genetic material in influenza virus?
A)
single-helix DNA
done
clear
B)
Double-helix DNA
done
clear
C)
RNA
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) The daughters born to haemophilic father and normal mother could be
A)
normal
done
clear
B)
carrier
done
clear
C)
haemophilic
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 126) Which of the following are initiator codons?
A)
UUU and UUC
done
clear
B)
UAA and UAG
done
clear
C)
UGA and UAG
done
clear
D)
AUG and GUG
done
clear
View Answer play_arrow
question_answer 127) The transfer of genetic material from one bacterium to another by virus is called
A)
transduction
done
clear
B)
translation
done
clear
C)
conjugation
done
clear
D)
transformation
done
clear
View Answer play_arrow
question_answer 128) The petiole modified into flattened lamina like structure is present in
A)
Acacia nilotica
done
clear
B)
Acacia Arabica
done
clear
C)
Acacia Australasia
done
clear
D)
Acacia anuriculoformis
done
clear
View Answer play_arrow
question_answer 129) Cyathium and hypanthodium resemble each other in possessing
A)
involucres
done
clear
B)
dioecious nature
done
clear
C)
receptacle
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 130) In a flowring plant, archesporium gives rise to
A)
only the wall of the sporangium
done
clear
B)
both wall and the sporogenous cells
done
clear
C)
wall and the tapetum
done
clear
D)
only tapetum and sporogenous cells
done
clear
View Answer play_arrow
question_answer 131) Seedless watermelons are obtained by
A)
triploidy
done
clear
B)
gibberellin application
done
clear
C)
haploidy
done
clear
D)
vegetative propagation
done
clear
View Answer play_arrow
question_answer 132) The female hormone inhibin is secreted by
A)
zona pelluida
done
clear
B)
ovary
done
clear
C)
corpus luteum
done
clear
D)
uterine epithelium
done
clear
View Answer play_arrow
question_answer 133) Which one of the following is not associated with the theory of ageing?
A)
Somatic mutation
done
clear
B)
Epimorphosis
done
clear
C)
Metabolic rate
done
clear
D)
Neurohormonal change
done
clear
View Answer play_arrow
question_answer 134) What justifies that Rafflesia exhibits dipteran entomophily?
A)
It look us like rotten meat
done
clear
B)
It is very large in size
done
clear
C)
It sinks live rotten meat
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 135) According to the law of population growth if an initial population of yeast cells 10 in number was allowed to grow for 6 hours, the expected number of individuals in the final population will be about
A)
600
done
clear
B)
100 only
done
clear
C)
50 only
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 136) In biotic community, which one of the following can be called protective device?
A)
Symbiosis
done
clear
B)
Mimicry
done
clear
C)
Competition
done
clear
D)
Parasitism
done
clear
View Answer play_arrow
question_answer 137) Biogeochemical cycle of which element has atmospheric phase?
A)
Carbon
done
clear
B)
Sodium
done
clear
C)
Phosphorus
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 138) The photovoltaic system helps in converting
A)
biogas
done
clear
B)
dendrothermal energy
done
clear
C)
ar energy
done
clear
D)
tidal energy
done
clear
View Answer play_arrow
question_answer 139) Animals like cockroach, lizards and mice share buildings to human de vellings Such animals are
A)
transgenic
done
clear
B)
transplants
done
clear
C)
induilines
done
clear
D)
cultigens
done
clear
View Answer play_arrow
question_answer 140) India is under which stage of demographic cycle?
A)
Early expanding
done
clear
B)
late expanding
done
clear
C)
Phase of decline
done
clear
D)
High stationary
done
clear
View Answer play_arrow
question_answer 141) Genetic drift operates only in
A)
island populations
done
clear
B)
smaller populations
done
clear
C)
larger populations
done
clear
D)
Mendelian populations
done
clear
View Answer play_arrow
question_answer 142) Most commonly used analgesic during self medication by persons is
A)
aspirin
done
clear
B)
ibuprofen
done
clear
C)
acetamirophen
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 143) Formation of alcohol by yeast is
A)
anaerobic process
done
clear
B)
extracellular
done
clear
C)
microbial
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 144) Broodiness in layer birds can be rectified by giving hormonal injection of
A)
progesterone
done
clear
B)
oestrogen
done
clear
C)
thyroxine
done
clear
D)
pituitary extract
done
clear
View Answer play_arrow
question_answer 145) The amount of protein per 100 g (without water, approximately 2 eggs) is
A)
119
done
clear
B)
201
done
clear
C)
16
done
clear
D)
45
done
clear
View Answer play_arrow
question_answer 146) Which of the following is exotic breed of cattle?
A)
Brown jersey
done
clear
B)
Friesion
done
clear
C)
Holstein
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 147) Which of the following is incorrectly matched?
A)
Organophosphate - Malathion
done
clear
B)
Carbamate - Carbofuran
done
clear
C)
Organochlorine - Dieldrin
done
clear
D)
Carbamate - Heptachlor
done
clear
View Answer play_arrow
question_answer 148) Which one is incorrect statement?
A)
Green manures are added to the soil
done
clear
B)
Fertilizers cause soil and water pollution
done
clear
C)
Biofertilizers improve soil fertility
done
clear
D)
Berseem is an ideal biofertilizer
done
clear
View Answer play_arrow
question_answer 149) Which of the following crops occupy the highest acreage in India?
A)
Rice
done
clear
B)
Bajra
done
clear
C)
Wheat
done
clear
D)
Jowar
done
clear
View Answer play_arrow
question_answer 150) ar energy transducer is
A)
Agaricus
done
clear
B)
Rhizobium
done
clear
C)
Orobanche
done
clear
D)
Chlorella
done
clear
View Answer play_arrow
question_answer 151) Brewers yeast lack which of these?
A)
Diastase and amylase
done
clear
B)
Aamylase only
done
clear
C)
Diastase only
done
clear
D)
Maltose
done
clear
View Answer play_arrow
question_answer 152) Cloning is meant for
A)
production of HaH gene is E coli
done
clear
B)
to preserve the genotype of organism
done
clear
C)
to replace the original gene
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 153) HIV mainly infects
A)
cytotoxic T- lymphocytes
done
clear
B)
helper lymphocytes
done
clear
C)
cell mediated T- lymphocytes
done
clear
D)
Hiller T- lymphocytes
done
clear
View Answer play_arrow
question_answer 154) The region in the body where the polio virus multiplies is
A)
nerve cells
done
clear
B)
muscle cells
done
clear
C)
intestinal cells
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) Diabetes insipidus occurs due to hyposecretion of
A)
oxytocin
done
clear
B)
vasopressin
done
clear
C)
thymosine
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 156) Animals virus contains mostly
A)
RNA
done
clear
B)
DNA
done
clear
C)
RNA or DNA
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 157) A haemophilic man marries a normal homozygous woman What is the probability that their son will be haemophilic?
A)
100%
done
clear
B)
75%
done
clear
C)
50%
done
clear
D)
0%
done
clear
View Answer play_arrow
question_answer 158) Positron is
A)
positivily charged electron
done
clear
B)
proton
done
clear
C)
neutron
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 159) The Piezo-electric effect is used in
A)
NMR
done
clear
B)
PET
done
clear
C)
sonography
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 160) The process in which the amount of DNA, RNA and protein can be known at a time is called
A)
autoradiography
done
clear
B)
tissue culture
done
clear
C)
phase- contrast microscopy
done
clear
D)
cellular fractioning
done
clear
View Answer play_arrow
question_answer 161) The wood used for making musical instruments as xylophone is
A)
Dalbergia
done
clear
B)
Tactona
done
clear
C)
Plywood
done
clear
D)
Santalum
done
clear
View Answer play_arrow
question_answer 162) Resupinate condition is characteristic of the flowers belonging to the most advanced family of monocots
A)
Palmae
done
clear
B)
Liliaceae
done
clear
C)
Orchidaceae
done
clear
D)
Gramineae
done
clear
View Answer play_arrow
question_answer 163) GA usually promotes
A)
sterlity in flowers
done
clear
B)
maleness in flowers
done
clear
C)
femaleness in flowers
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 164) The most essential precusor of ethylene is
A)
adenine
done
clear
B)
thiocarbamate
done
clear
C)
zearin
done
clear
D)
methionine
done
clear
View Answer play_arrow
question_answer 165) At which stage the enzymatic actions start in plant?
A)
Germination
done
clear
B)
At the time of photosynthesis
done
clear
C)
At the time of flower establishment
done
clear
D)
At the time of fertilization
done
clear
View Answer play_arrow
question_answer 166) A non - sense mutation results into
A)
stoppage of transcription
done
clear
B)
change in protein structure
done
clear
C)
stoppage of protein synthesis
done
clear
D)
termination of polypeptide chain
done
clear
View Answer play_arrow
question_answer 167) A polysome is formed by
A)
a cluster of ribosomes
done
clear
B)
many ribosomes attached to a mRNA
done
clear
C)
a cluster of ribosomal sub units
done
clear
D)
many mRNAs being attached to a ribosome
done
clear
View Answer play_arrow
question_answer 168) Which of the following pairs is correctly matched?
A)
RNA polymerase - RNA primer
done
clear
B)
Okazaki fragments - splicing
done
clear
C)
Restriction enzymes - Genetic engineering
done
clear
D)
Central dogma - codon
done
clear
View Answer play_arrow
question_answer 169) Gene TDF occurs in
A)
Y-chromosome
done
clear
B)
X-chromosome
done
clear
C)
Both X and Y-chromosomes
done
clear
D)
autosomes
done
clear
View Answer play_arrow
question_answer 170) Asafoetida is a
A)
tannin
done
clear
B)
alkaloid
done
clear
C)
resin
done
clear
D)
oil
done
clear
View Answer play_arrow
question_answer 171) The leaves without petiole are called
A)
lamina
done
clear
B)
rachis
done
clear
C)
petiolate
done
clear
D)
sessile
done
clear
View Answer play_arrow
question_answer 172) In Gloriosa, the tendrillar part is formed by
A)
stipule
done
clear
B)
leaf apex
done
clear
C)
leaf petiole
done
clear
D)
axillary bud
done
clear
View Answer play_arrow
question_answer 173) Cleistogamous flowers
A)
open at dusk
done
clear
B)
open at down
done
clear
C)
open during noon
done
clear
D)
never open
done
clear
View Answer play_arrow
question_answer 174) Syngenecious condition means
A)
anthers fused, filaments free
done
clear
B)
both anthers and filaments used
done
clear
C)
filaments fused and anthers free
done
clear
D)
filaments fused with carpals
done
clear
View Answer play_arrow
question_answer 175) The specialized tissue includes
A)
sclereid
done
clear
B)
sclerenchyma
done
clear
C)
nectarines
done
clear
D)
collenchymas
done
clear
View Answer play_arrow
question_answer 176) Casparian strips occur in
A)
radial walls of endodermis
done
clear
B)
radial walls of phellem cells
done
clear
C)
radial walls of epidermal cells
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 177) When sunflower leaf is exposed to sunlight, the ar radiations first pass through its upper epidermis and then through
A)
spongy parenchyma
done
clear
B)
lower epidermis
done
clear
C)
vascular bundles
done
clear
D)
palisade parenchyma
done
clear
View Answer play_arrow
question_answer 178) Piliferous layer in root is
A)
epidermis
done
clear
B)
pericycle
done
clear
C)
cortex
done
clear
D)
endoderics
done
clear
View Answer play_arrow
question_answer 179) Which pair is incorrect?
A)
Patella - knee cap
done
clear
B)
Malleus - hammer bone
done
clear
C)
Sternum - chest bone
done
clear
D)
Stapes - anvil bone
done
clear
View Answer play_arrow
question_answer 180) Osteoblasts are found in
A)
blood
done
clear
B)
muscle
done
clear
C)
bone
done
clear
D)
cartilage
done
clear
View Answer play_arrow
question_answer 181) Assertion Bats and whales are classified as mammals Reason (R) Bats and whales have- four-chambered heart
A)
A is correct and R is its explanation
done
clear
B)
A is correct and R is also correct but R is not explanation to A
done
clear
C)
A is correct and R is wrong
done
clear
D)
A and R both are wrong
done
clear
View Answer play_arrow
question_answer 182) The only poisonous lizard of the world is
A)
Hemidactylns
done
clear
B)
Phrynosoma
done
clear
C)
Heloderma
done
clear
D)
Uromastix
done
clear
View Answer play_arrow
question_answer 183) Corpus luteum is developed from
A)
left over occyte
done
clear
B)
nephrostome
done
clear
C)
left over Graafian follicle after release of ovum
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 184) Which one of the following is not an example of accretionary growth?
A)
Increase in the size of muscles due to regular exercise
done
clear
B)
Prenatal growth of the embryo
done
clear
C)
Both of the above
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 185) The yolk of avian eggs contain
A)
albumin
done
clear
B)
provitallin
done
clear
C)
vitellin
done
clear
D)
leuthin
done
clear
View Answer play_arrow
question_answer 186) One of the following controls the population from going beyond its limit
A)
Carrying capacity
done
clear
B)
Environmental resistance
done
clear
C)
Family planning programme
done
clear
D)
Shortage of food supply
done
clear
View Answer play_arrow
question_answer 187) The rejection of organ transplanting in humans is prevented by using
A)
asprin
done
clear
B)
cyclosporine
done
clear
C)
calsitonin
done
clear
D)
thrombin
done
clear
View Answer play_arrow
question_answer 188) interferons act against
A)
bacteria
done
clear
B)
virus
done
clear
C)
Protozoa
done
clear
D)
bacteriophage
done
clear
View Answer play_arrow
question_answer 189) Wilting occurs due to excessive
A)
transpiration
done
clear
B)
respiration
done
clear
C)
guttation
done
clear
D)
absorption
done
clear
View Answer play_arrow
question_answer 190) The shade of a tree is cooler than the shade of a roof due to
A)
guttation
done
clear
B)
green leaves
done
clear
C)
transpiration
done
clear
D)
photosynthesis
done
clear
View Answer play_arrow
question_answer 191) The haemoglobin like pigment can be traced in
A)
seeds of legumes
done
clear
B)
Clostridium
done
clear
C)
Rhizobium
done
clear
D)
leguminous root nodules
done
clear
View Answer play_arrow
question_answer 192) \[{{C}_{4}}\]plants, the primary \[C{{O}_{2}}\]acceptor is
A)
3- PGA
done
clear
B)
oxalo acetic acid
done
clear
C)
RuBP
done
clear
D)
PEP
done
clear
View Answer play_arrow
question_answer 193) Largest amount of phosphate bond energy is produced in the process of respiration during
A)
glycosis
done
clear
B)
Krebs cycle
done
clear
C)
anaerobic respiration
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 194) Root pressure has never been observed in
A)
Pteridophytes
done
clear
B)
Gymnosperms
done
clear
C)
Monocotyledons
done
clear
D)
Dicotyledons
done
clear
View Answer play_arrow
question_answer 195) In respect of many grasses, the presence of motor cells in the upper epidermis of leaves is to
A)
increase the surface area of the leaf
done
clear
B)
store large amounts of water
done
clear
C)
check transpiration by reducing the surface of the leaf
done
clear
D)
bear unicellular trichomes
done
clear
View Answer play_arrow
question_answer 196) Camouflage of chamaeleon is associated with
A)
chromosome
done
clear
B)
chromomere
done
clear
C)
chromoplast
done
clear
D)
chromatophore
done
clear
View Answer play_arrow
question_answer 197) What would happen if fangs of venomous snake are pulled out?
A)
They will get replaced
done
clear
B)
They will become double
done
clear
C)
They will be safe to handle
done
clear
D)
They will never get replaced
done
clear
View Answer play_arrow
question_answer 198) Diapedesis is
A)
formation of pus
done
clear
B)
bursting of WBCs
done
clear
C)
formation of WBCs
done
clear
D)
passage of WBCs
done
clear
View Answer play_arrow
question_answer 199) Brain and spinal cord are
A)
intermediary neurons
done
clear
B)
effectors
done
clear
C)
receptors
done
clear
D)
sensory organs
done
clear
View Answer play_arrow
question_answer 200) In mammals caecum
A)
is a reservoir of bacteria
done
clear
B)
provides additional space for colonic bacteria
done
clear
C)
distal end is degenerated, remnant being represented by appendix
done
clear
D)
All of the above
done
clear
View Answer play_arrow
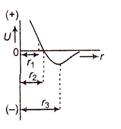 Read the following statements carefully The equilibrium separation distance between the atoms is equal to \[{{r}_{2}}\] At \[\text{r}={{\text{r}}_{\text{1}}},\]the force between the atoms is repulsive For \[\begin{align} & \text{V}{{\text{p}}^{\text{2}}}= \\ & \\ \end{align}\] the force between the atoms is attractive TO. Which of the above statements is true?
Read the following statements carefully The equilibrium separation distance between the atoms is equal to \[{{r}_{2}}\] At \[\text{r}={{\text{r}}_{\text{1}}},\]the force between the atoms is repulsive For \[\begin{align} & \text{V}{{\text{p}}^{\text{2}}}= \\ & \\ \end{align}\] the force between the atoms is attractive TO. Which of the above statements is true?
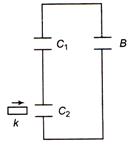 (i) increase in the value of charge on \[{{C}_{1}}\] (ii) increase in the value of charge on \[{{C}_{2}}\] (iii) increase in the value of potential difference across the plates of \[{{C}_{1}}\] (iv) decrease in the value of potential difference across the plates of \[{{C}_{3}}\] Which of the above is true?
(i) increase in the value of charge on \[{{C}_{1}}\] (ii) increase in the value of charge on \[{{C}_{2}}\] (iii) increase in the value of potential difference across the plates of \[{{C}_{1}}\] (iv) decrease in the value of potential difference across the plates of \[{{C}_{3}}\] Which of the above is true?
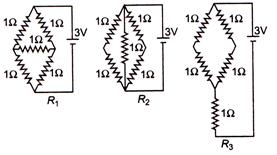 If the powers dissipated by the configurations \[{{R}_{1}},\text{ }{{R}_{2}}\]and \[{{R}_{3}}\]are \[{{P}_{1}},\text{ }{{P}_{2}}\]and \[{{P}_{3}},\]respectively, then
If the powers dissipated by the configurations \[{{R}_{1}},\text{ }{{R}_{2}}\]and \[{{R}_{3}}\]are \[{{P}_{1}},\text{ }{{P}_{2}}\]and \[{{P}_{3}},\]respectively, then
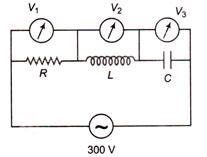
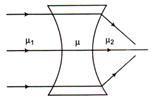
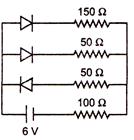
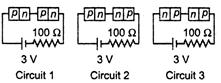 The potential drops across the two \[p-n\] junctions are equal in
The potential drops across the two \[p-n\] junctions are equal in
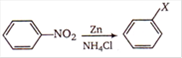 X stands for
X stands for