question_answer 1)
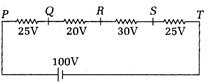
The potential differences across the resistances are given in the following circuit as shown. If point Q is grounded, the potential at point S will be
A)
20V
done
clear
B)
-20V
done
clear
C)
50V
done
clear
D)
-50V
done
clear
View Answer play_arrow
question_answer 2)
A straight conductor carrying a current I splits into two identical semicircular arcs as shown in figure below. What is the magnitude of the magnetic field at the centre C of the resulting circular arcs of radius R?
A)
0
done
clear
B)
\[\frac{{{\mu }_{0}}l}{4R}\]
done
clear
C)
\[\frac{{{\mu }_{0}}l}{2R}\]
done
clear
D)
\[\frac{{{\mu }_{0}}l}{R}\]
done
clear
View Answer play_arrow
question_answer 3) A circular loop of area 1\[c{{m}^{2}}\], carrying a current of 10 A is placed in a magnetic field of T perpendicular to the plane of the loop. The torque on the loop due to the magnetic field is
A)
zero
done
clear
B)
\[{{10}^{-4}}N\,m\]
done
clear
C)
\[{{10}^{-2}}N\,m\]
done
clear
D)
1 Nm
done
clear
View Answer play_arrow
question_answer 4) An average of emf 20 V is induced in an inductor when the current I in it is changed from 2.5 A in one direction to the same value in the opposite direction in 0.1 s. What is the self-inductance of the inductor?
A)
Zero
done
clear
B)
0.2 H
done
clear
C)
0.4H
done
clear
D)
0.1H
done
clear
View Answer play_arrow
question_answer 5)
Two cells of emf \[{{E}_{1}}\] and \[{{E}_{2}}({{E}_{1}}>{{E}_{2}})\] are connected as shown
A)
3/2
done
clear
B)
2/3
done
clear
C)
1/2
done
clear
D)
1/3
done
clear
View Answer play_arrow
question_answer 6) A convex lens is made of a material having refractive index 1.2. Both the sides are convex. If it is dipped in water (27 = 1.3 3), it will behave like a
A)
convergent lens
done
clear
B)
divergept lens
done
clear
C)
a rectangular slab
done
clear
D)
a prisms
done
clear
View Answer play_arrow
question_answer 7) What will be the angle of diffraction for the first secondary maximum due to diffraction at a single slit of width 0.4 mm, using light of wavelength 4000 Å?
A)
\[1.0\times {{10}^{-4}}\] rad
done
clear
B)
\[0.5\times {{10}^{-4}}\] rad
done
clear
C)
\[0.15\times {{10}^{-3}}\] rad
done
clear
D)
\[1.5\times {{10}^{-3}}\] rad
done
clear
View Answer play_arrow
question_answer 8) A glass slab of thickness 8 cm contains the same number of waves as 10 cm of water when both are traversed by the same monochromatic light. If the refractive index of water is 4/3, the refractive index of glass is
A)
3/4
done
clear
B)
3/5
done
clear
C)
5/3
done
clear
D)
5/4
done
clear
View Answer play_arrow
question_answer 9) If E and B are the electric and magnetic fields vectors of electromagnetic waves then the direction of propagation of electromagnetic waves is along the direction of
A)
E
done
clear
B)
B
done
clear
C)
\[E\times B\]
done
clear
D)
\[B\times E\]
done
clear
View Answer play_arrow
question_answer 10) The amplitude of the magnetic field of a harmonic electromagnetic wave in vacuum I Bo = 510 nT. What is the amplitude of the electric field?
A)
140 \[N{{C}^{-1}}\]
done
clear
B)
153 \[N{{C}^{-1}}\]
done
clear
C)
163 \[N{{C}^{-1}}\]
done
clear
D)
133 \[N{{C}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 11) lie A free electron is placed in the path of a plane electromagnetic wave. The electron will start moving
A)
along the electric field
done
clear
B)
along the magnetic field
done
clear
C)
along the direction of propagation of wave
done
clear
D)
in a plane containing the magnetic field and the direction of propagation
done
clear
View Answer play_arrow
question_answer 12) Ultraviolet light of wavelength 350 nm and intensity \[1.00\text{ }W/{{m}^{2}}\]is directed at a potassium surface. What will be the maximum kinetic energy of the photoelectrons? (Work function of potassium =2.2eV)
A)
3.5eV
done
clear
B)
2.6eV
done
clear
C)
2.2eV
done
clear
D)
1.3eV
done
clear
View Answer play_arrow
question_answer 13) Light of wavelength \[\lambda =5893\,\overset{o}{\mathop{A}}\,\] is incident on potassium surface. The stopping potential for the emitted electrons is 0.36 V. What is the maximum kinetic energy of the photoelectrons?
A)
0.18eV
done
clear
B)
0.36eV
done
clear
C)
0.74eV
done
clear
D)
2.1eV
done
clear
View Answer play_arrow
question_answer 14) If the kinetic energy of a particle is reduced to one - fourth then the percentage increase in the de - Broglie wavelength is
A)
41%
done
clear
B)
100%
done
clear
C)
144%
done
clear
D)
200%
done
clear
View Answer play_arrow
question_answer 15) In the Bohr Model of hydrogen atom, the electron circulates around the nucleus in a path of radius \[5.1\times {{10}^{-11}}\] m at a frequency of \[6.8\times {{10}^{15}}\] revolutions per second. What will be the equivalent current?
A)
2.544 mA
done
clear
B)
2.176 mA
done
clear
C)
1.880 mA
done
clear
D)
1.088 mA
done
clear
View Answer play_arrow
question_answer 16) The graph of log \[(R/{{R}_{0}})\] versus log A (R = radius of a nucleus and A = its mass number) is
A)
a straight line
done
clear
B)
a parabola
done
clear
C)
an ellipse
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 17) An \[\alpha \]-particle is bombarded on \[^{14}N\]. As a result, a \[^{17}O\] nucleus is formed and a particle is emitted. This particle is a/an
A)
neutron
done
clear
B)
proton
done
clear
C)
electron
done
clear
D)
positron
done
clear
View Answer play_arrow
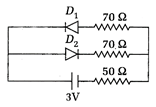
question_answer 18)
The circuit shown in the diagram contains two diodes, each with a forward resistance of 30 \[\Omega \]and infinite reverse resistance. If the battery is of 3 V, then the voltage drop across 50 \[\Omega \]resistance will be
A)
2V
done
clear
B)
5 V
done
clear
C)
1 V
done
clear
D)
1.5V
done
clear
View Answer play_arrow
question_answer 19) The depletion layer in the p-n junction region is caused by
A)
drift of holes
done
clear
B)
diffusion of charge carriers
done
clear
C)
migration of impurity ions
done
clear
D)
drift of electrons
done
clear
View Answer play_arrow
question_answer 20) In order to be transmitted over a communication system, a speech signal with frequencies in the range 0.2-5 kHz is amplitude modulated over a carrier wave of frequency 1000 kHz. What would be the maximum transmitted frequency?
A)
5 kHz
done
clear
B)
1000 kHz
done
clear
C)
1000.2 kHz
done
clear
D)
1005 kHz
done
clear
View Answer play_arrow
question_answer 21) The dimensional formula of Plancks constant, h is
A)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{0}}{{T}^{2}}]\]
done
clear
D)
\[[ML{{T}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 22) Astronomical unit (Au) is the average distance between the earth and sun approximately \[1.5\times {{10}^{8}}km\]. The speed of light is about\[3.0\times {{10}^{8}}m/s\]. The speed of light in astronomical unit per minute is
A)
0.012 Au/min
done
clear
B)
0.12 Au/min
done
clear
C)
1.2 Au/min
done
clear
D)
12.0 Au/min
done
clear
View Answer play_arrow
question_answer 23) A 130 m long train is moving in up direction with speed 17 km/h. Another train of 120 m long is moving in down direction with speed 108 km/h. The time in which second train crosses the first train will be
A)
5s
done
clear
B)
10s
done
clear
C)
12s
done
clear
D)
15s
done
clear
View Answer play_arrow
question_answer 24) At a certain time a particle has a speed of 18 m/s in positive x-direction and 2.4 s later its speed was 30 m/s in the opposite direction. What is the magnitude of the average acceleration of the particle during the 2.4 s interval?
A)
\[20\,m/{{s}^{2}}\]
done
clear
B)
\[10\,m/{{s}^{2}}\]
done
clear
C)
\[5\,m/{{s}^{2}}\]
done
clear
D)
\[2.5\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
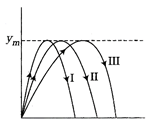
question_answer 25)
Three projectile motion paths are shown below. Each having reached the same height\[{{y}_{m}}\]. For which path the time of flight is minimum.
A)
I
done
clear
B)
II
done
clear
C)
III
done
clear
D)
All have the same time of flight
done
clear
View Answer play_arrow
question_answer 26) A certain force gives an object of mass \[{{m}_{2}}\] an acceleration of \[12\,\,m/{{s}^{2}}\] and an object of mass \[{{m}_{2}}\] an acceleration of \[3\,\,m/{{s}^{2}}\]. What acceleration would the force give to an object of mass \[{{m}_{2}}={{m}_{1}}\]?
A)
\[0.5\,m/{{s}^{2}}\]
done
clear
B)
\[1.0\,m/{{s}^{2}}\]
done
clear
C)
\[2.0\,m/{{s}^{2}}\]
done
clear
D)
\[4.0\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27)
Two blocks A and B of masses 10 kg and 20 kg respectively are connected by a massless spring as shown in figure. A force of 200 N acts on the block B. At the instant shown, the block A has an acceleration of \[12\,m/{{s}^{2}}\]The acceleration of the block B will be
A)
zero
done
clear
B)
\[4\,m/{{s}^{2}}\]
done
clear
C)
\[10\,m/{{s}^{2}}\]
done
clear
D)
\[12\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 28) A body of mass 10 kg moves at a constant speed of \[10\,m/{{s}^{2}}\]. A constant force then acts for 4 second on the body and gives it a speed of 2 m/s in opposite direction. Magnitude of the force acting on the body is
A)
30N
done
clear
B)
20N
done
clear
C)
15N
done
clear
D)
10N
done
clear
View Answer play_arrow
question_answer 29) A stone is fastened to one end of a string and is whirled in a vertical circle of radius R. Find the minimum speed the stone can have at the highest point of the circle.
A)
\[\sqrt{2Rg}\]
done
clear
B)
\[\sqrt{2R/g}\]
done
clear
C)
\[\sqrt{R/g}\]
done
clear
D)
\[\sqrt{Rg}\]
done
clear
View Answer play_arrow
question_answer 30) If g is acceleration due to gravity on earths surface, the gain in the potential energy of an object of mass m raised from surface of the earth to a height equal to radius R of the earth is
A)
\[2\,mg\,R\]
done
clear
B)
\[\,mg\,R\]
done
clear
C)
\[\frac{1}{2}\,mg\,R\]
done
clear
D)
\[\frac{1}{4}\,mg\,R\]
done
clear
View Answer play_arrow
question_answer 31) A man racing with his son has half the kinetic energy of the son, who has half the mass of the father. The man speeds up by 1 m/s and has the same kinetic energy as the son. What was the original speed of the man?
A)
4.8 m/s
done
clear
B)
3.6 m/s
done
clear
C)
2.4 m/s
done
clear
D)
1.2 m/s
done
clear
View Answer play_arrow
question_answer 32) All particles of a body are situated at a distance R from the origin. The distance of the centre of mass of the body from the origin is
A)
= R
done
clear
B)
\[\le R\]
done
clear
C)
> R
done
clear
D)
\[\ge R\]
done
clear
View Answer play_arrow
question_answer 33) A wheel is rotating freely at angular speed 800 rev/min on a shaft whose rotational inertia negligible. A second wheel, initially at rest and with twice the rotational inertia of the first is suddenly coupled to the shaft. The angular speed of the resultant combination of the shaft and two wheel is
A)
267 rev/min
done
clear
B)
335 rev/min
done
clear
C)
400 rev/min
done
clear
D)
1150 rev/min
done
clear
View Answer play_arrow
question_answer 34) A person sitting firmly over a rotating stool has his arms stretched. If he folds his arms, his angular momentum about the axis of rotation
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchanged
done
clear
D)
doubles
done
clear
View Answer play_arrow
question_answer 35) An asteroid, whose mass is \[2.0\times {{10}^{-4}}\] times the mass of Earth, revolves in a circular orbit around the sun at a distance that is twice the Earths distance form the sun. The period of revolution of asteroid in years is (mass of the sun \[=1.99\times {{10}^{30}}kg\], radius of the Earths orbit \[=1.5\times {{10}^{11}}m\],\[G=6.67\times {{10}^{-11}}{{m}^{3}}/{{s}^{2}}\,kg\])
A)
0.28 yr
done
clear
B)
2.8 yr
done
clear
C)
10yr
done
clear
D)
28 yr
done
clear
View Answer play_arrow
question_answer 36) The pressure increases on the fluid in a syringe when a nurse applies a force of 42 N to the syringes circular piston, which has a radius of 1.1 cm.
A)
\[1.1\times {{10}^{5}}Pa\]
done
clear
B)
\[11\times {{10}^{5}}Pa\]
done
clear
C)
\[1.1\times {{10}^{7}}Pa\]
done
clear
D)
\[1.1\times {{10}^{1}}Pa\]
done
clear
View Answer play_arrow
question_answer 37) A solid sphere falls with a terminal velocity of 20 m/s in air. If it is allowed to fall in vacuum?
A)
the terminal velocity will be 20 m/s
done
clear
B)
the terminal velocity will be less than 20 m/s
done
clear
C)
the terminal velocity will be more than 20 m/s
done
clear
D)
there will be no terminal velocity
done
clear
View Answer play_arrow
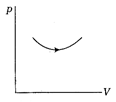
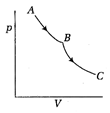
question_answer 38)
Consider the process on a system shown in figure below. During the process, the work done by the system
A)
continuously increases
done
clear
B)
continuously decreases
done
clear
C)
first increases then decreases
done
clear
D)
first decreases then increases
done
clear
View Answer play_arrow
question_answer 39)
An ideal gas is taken from state A to state B and then from state B to state C as shown in figure. Which of the following statements is true?
A)
Process AB is isochoric, while process BC is isobaric
done
clear
B)
Process AB is isobaric, while process BC is isochoric
done
clear
C)
Process AB is isothermal while process BC is adiabatic
done
clear
D)
Process AB is adiabatic while process BC is isothermal
done
clear
View Answer play_arrow
question_answer 40) Two particles execute SHM of the same amplitude and frequency along the same line. They pass one another when going in opposite directions each time their displacement is half their amplitude. What is the phase difference between them?
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{120}^{o}}\]
done
clear
C)
\[{{160}^{o}}\]
done
clear
D)
\[{{180}^{o}}\]
done
clear
View Answer play_arrow
question_answer 41) A jet engine emits a sound of frequency 3000 Hz. When the engine is moving directly away from the observer at half the speed of sound (speed of sound = 340 m/s), the observer hears a sound of frequency
A)
1000 Hz
done
clear
B)
1500 Hz
done
clear
C)
2000 Hz
done
clear
D)
4500 Hz
done
clear
View Answer play_arrow
question_answer 42) A particle is executing SHM with a period \[\pi \]sec. When it is passing through the centre of its path, its velocity is 0.1 m/s. What is its velocity when it is at a distance of 0.03 m from the mean position?
A)
0.08 m/s
done
clear
B)
0.05 m/s
done
clear
C)
0.04 m/s
done
clear
D)
0.01 m/s
done
clear
View Answer play_arrow
question_answer 43) A proton is released from rest in a uniform electric field of magnitude \[8.0\times {{10}^{4}}\] V/m, directed along positive x-axis. The proton undergoes a displacement of 0.30m in the direction of the field. What is the change in electric potential of the proton as a result of this displacement?
A)
\[2.4\times {{10}^{4}}\,V\]
done
clear
B)
\[4.8\times {{10}^{4}}\,V\]
done
clear
C)
\[-2.4\times {{10}^{4}}\,V\]
done
clear
D)
\[-1.2\times {{10}^{4}}\,V\]
done
clear
View Answer play_arrow
question_answer 44) A positive point charge is brought near an isolated metal cube.
A)
The cubes becomes negatively charged
done
clear
B)
The cube becomes positively charged
done
clear
C)
The interior becomes positively charged and the surface becomes negatively charged
done
clear
D)
The interior remains charge free and the surface gets non-uniform charge distribution.
done
clear
View Answer play_arrow
question_answer 45) A charge Q is to be divided on two objects. The values of the charges on the objects so that the force between the objects can be maximum are
A)
\[\frac{2Q}{3},\frac{Q}{3}\]
done
clear
B)
\[\frac{3Q}{4},\frac{Q}{4}\]
done
clear
C)
\[\frac{Q}{2},\frac{Q}{2}\]
done
clear
D)
Q. O
done
clear
View Answer play_arrow
question_answer 46) Initially, sphere A has charge of-50 e and sphere B has a charge of +20e. The spheres are made of conducting material and are identical in size. If the spheres touch each other, what is the resulting charge on sphere A?
A)
-15e
done
clear
B)
-30e
done
clear
C)
+30e
done
clear
D)
+15e
done
clear
View Answer play_arrow
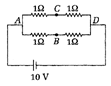
question_answer 47)
A battery of emf 10 V is connected to the network as shown in figure The potential difference between A and B is
A)
-2V
done
clear
B)
2V
done
clear
C)
5V
done
clear
D)
7V
done
clear
View Answer play_arrow
question_answer 48) The net resistance of an ammeter should be small to ensure that
A)
it does not get overheated
done
clear
B)
it does not draw excessive current
done
clear
C)
it can measure large currents
done
clear
D)
it does not appreciably change the current to be measured
done
clear
View Answer play_arrow
question_answer 49) Suppose the first strip A is red, second strip B is yellow, third strip C is orange and fourth strip .R is silver, from left to right, m a colour coded carbon resistor. What is the value of resistance?
A)
\[(24\pm 5%)\,k\Omega \]
done
clear
B)
\[(24\pm 10%)\,k\Omega \]
done
clear
C)
\[(350\pm 5%)\,k\Omega \]
done
clear
D)
\[(350\pm 10%)\,k\Omega \]
done
clear
View Answer play_arrow
question_answer 50) Two bulbs of rating 100 W, 250 V and 200 W, 250 V are connected in series across a 500 V line, then
A)
100 W bulb will be fused
done
clear
B)
200 W bulb will be fused
done
clear
C)
both bulbs will be fused
done
clear
D)
No bulb will be fused
done
clear
View Answer play_arrow
question_answer 51) Which one of the following is an addition polymer?
A)
Nylon-6
done
clear
B)
Nylon-6 6
done
clear
C)
Terylene (dacron)
done
clear
D)
Buna-S
done
clear
View Answer play_arrow
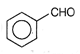
question_answer 52) Toluene on treatment with chromyl chloride in presence of \[C{{S}_{2}}\] yields a complex which upon hydrolysis yields
A)
benzole acid
done
clear
B)
acetophenone
done
clear
C)
benzaidehyde
done
clear
D)
benzyl alcohol
done
clear
View Answer play_arrow
question_answer 53) Which one of the following organic compound is a gas?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{2}}O\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 54) Lucas reagent is equimolar solution of
A)
cone. \[{{H}_{2}}S{{O}_{4}}\] and cone. \[HN{{O}_{3}}\]
done
clear
B)
cone. \[HN{{O}_{3}}\] and anhydrous \[ZnC{{l}_{3}}\]
done
clear
C)
cone. \[HCl\] and anhydrous \[ZnC{{l}_{2}}\]
done
clear
D)
cone. \[{{H}_{2}}S{{O}_{4}}\] and anhydrous \[ZnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 55) The carbocation formed in \[{{S}_{N}}1\] reaction of alkyl halide in the slow step is
A)
\[s{{p}^{3}}\] hybridised
done
clear
B)
\[s{{p}^{2}}\]hybridized
done
clear
C)
sp hybridised
done
clear
D)
\[s{{p}^{3}}d\] hybridised
done
clear
View Answer play_arrow
question_answer 56) The conversion of nitriles to aldehydes with stannous chloride in presence of \[HCl\] is known as
A)
Rosenmund reduction
done
clear
B)
Stephen reduction
done
clear
C)
Etard reaction
done
clear
D)
Wolf Kishner reduction
done
clear
View Answer play_arrow
question_answer 57) The Nobel Prize in Chemistry in 2005 was awarded for the work
A)
concerning the reduction of hazardous waster in creating new chemicals
done
clear
B)
concerning the formation and decomposition of ozone
done
clear
C)
concerning the recycling of CFCs
done
clear
D)
concerning the climate change
done
clear
View Answer play_arrow
question_answer 58) For a weak electrolyte, a plot of a (degree of ionisation)vs 1/C gives
A)
a straight line
done
clear
B)
a parabola
done
clear
C)
a hyperbola
done
clear
D)
an exponential curve
done
clear
View Answer play_arrow
question_answer 59) The pH of a solution obtained by mixing of 100 mL of a \[HCl\] solution of pH = 2 with 400 mL of another \[HCl\] solution of pH = 3 will be
A)
2
done
clear
B)
3
done
clear
C)
2.5
done
clear
D)
2.8
done
clear
View Answer play_arrow
question_answer 60) The reduced volume and reduced temperature of a gas are 10.2 and 0.7 respectively. If its critical pressure is 4.25 atm then its pressure will be
A)
0.6816 atm
done
clear
B)
0.6618 atm
done
clear
C)
0.8616 atm
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 61) For the electrode \[{{H}^{+}}(aq)\,\left| {{H}_{2}}(g) \right.\], if pH is decreased by one unit at \[{{25}^{o}}C\], then the cell potential
A)
decreases by 59.1 mV
done
clear
B)
increases by 59.1 mV
done
clear
C)
remains unchanged
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 62) For a reaction,\[NO\,(g)+{{O}_{2}}(g)\xrightarrow{{}}2N{{O}_{2}}\,(g);\] Rate \[=kl\,{{[NO]}^{2}}[{{O}_{2}}]\]. If the volume of the reaction vessel is doubled, the rate of reaction
A)
will diminish to 1/4 of initial value
done
clear
B)
will diminish to 1/8 of initial value
done
clear
C)
will increase 4 times
done
clear
D)
will increase 8 times
done
clear
View Answer play_arrow
question_answer 63) Two moles of an ideal gas are allowed to expand reversibly and isothermally at 300 K from a pressure of 1 atm to a pressure of 0.1 atm. The change in Gibbs free energy is
A)
-11.488J
done
clear
B)
+11.488J
done
clear
C)
-11.488kJ
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 64) What will be pH of the following half-cell\[Pt,\,{{H}_{2}}\left| {{H}_{2}}S{{O}_{4}} \right.\]? The oxidation potential is + 0.3V.
A)
3.085
done
clear
B)
4.085
done
clear
C)
5.085
done
clear
D)
6.085
done
clear
View Answer play_arrow
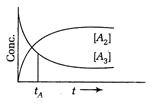
question_answer 65)
Consider a first order decomposition process \[{{A}_{3}}\xrightarrow{{}}\frac{3}{2}{{A}_{2}}\]A plot of concentration of \[{{A}_{3}}\] and \[{{A}_{2}}\] versus time is shown below. At time \[{{t}_{A}}\], percentage of reactant decomposed is
A)
75%
done
clear
B)
50%
done
clear
C)
40%
done
clear
D)
30%
done
clear
View Answer play_arrow
question_answer 66) The composition of a sample of website is\[F{{e}_{0.95}}{{O}_{1.00}}\]. What percentage of iron is present in the form of Fe (III)?
A)
16.05
done
clear
B)
15.05
done
clear
C)
18.05
done
clear
D)
17.05
done
clear
View Answer play_arrow
question_answer 67) How many grams of \[KCl\] should be added to kg of water to lower its freezing point to \[{{8.0}^{o}}C\]? [\[{{K}_{f}}\] for water \[={{1.86}^{o}}C\,k\,g\,mo{{l}^{-1}}\])
A)
160.2
done
clear
B)
150.2
done
clear
C)
140.2
done
clear
D)
130.2
done
clear
View Answer play_arrow
question_answer 68) 0.01 m solution of \[KCl\] and \[BaC{{l}_{2}}\] are prepared in water. The freezing point of \[KCl\] found to be \[-{{4}^{o}}C\]. What will be the freezing point for \[BaC{{l}_{2}}\] solution assuming that both \[KCl\] and \[BaC{{l}_{2}}\] are completely ionised in solutions?
A)
\[-{{3}^{o}}C\]
done
clear
B)
\[-{{4}^{o}}C\]
done
clear
C)
\[-{{5}^{o}}C\]
done
clear
D)
\[-{{6}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 69) The emf of the following three cells I. \[Zn\left| Z{{n}^{2+}}\,\,(1M) \right|\left| C{{u}^{2}}(1M)\,\, \right|Cu\] II. \[Zn\left| Z{{n}^{2+}}\,\,(0.1M) \right|\left| C{{u}^{2}}(1M)\,\, \right|Cu\] III. \[Zn\left| Z{{n}^{2+}}\,\,(1M) \right|\left| C{{u}^{2+}}(0.1M)\,\, \right|Cu\] are represented by \[{{E}_{1}},{{E}_{2}}\] and \[{{E}_{3}}\]. Which of the following statement is correct?
A)
\[{{E}_{1}}>{{E}_{2}}>{{E}_{3}}\]
done
clear
B)
\[{{E}_{3}}>{{E}_{2}}>{{E}_{1}}\]
done
clear
C)
\[{{E}_{3}}>{{E}_{1}}>{{E}_{2}}\]
done
clear
D)
\[{{E}_{2}}>{{E}_{1}}>{{E}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) 20 \[d{{m}^{3}}\] of \[S{{O}_{2}}\] diffuse through a porous partition in 60 s. What volume of \[{{O}_{3}}\] will diffuse under similar conditions?
A)
14.1 \[d{{m}^{3}}\]
done
clear
B)
16.1 \[d{{m}^{3}}\]
done
clear
C)
18.1 \[d{{m}^{3}}\]
done
clear
D)
20.1 \[d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 71) What will be the pH of 0.1 M ammonium acetate solution? \[(p{{K}_{a}}=p{{K}_{b}}=4.74)\]
A)
4.74
done
clear
B)
6.74
done
clear
C)
7.74
done
clear
D)
7.00
done
clear
View Answer play_arrow
question_answer 72) The solubility of \[Sr{{(OH)}_{2}}\] at 298 K is 0.1581 M. What will be the concentration of \[{{H}_{3}}{{O}^{+}}\] in the solution?
A)
\[0.158\times {{10}^{-14}}\]
done
clear
B)
\[0.3162\times {{10}^{-14}}\]
done
clear
C)
\[3.163\times {{10}^{-14}}\]
done
clear
D)
\[2.163\times {{10}^{-14}}\]
done
clear
View Answer play_arrow
question_answer 73) The \[{{K}_{p}}\] value for the reaction,\[{{H}_{2}}(g)+{{I}_{2}}(g)2HI(g)\] at \[{{460}^{o}}C\] is 49. If the partial pressure of \[{{H}_{2}}\] and \[{{I}_{2}}\] is 0.5 atm, respectively. What will be the partial pressure of HI at equilibrium?
A)
0.389
done
clear
B)
0.111
done
clear
C)
0.788
done
clear
D)
0,222
done
clear
View Answer play_arrow
question_answer 74) Enthalpy of \[C{{H}_{4}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{H}_{3}}OH\], is negative. If enthalpies of combustion of \[C{{H}_{4}}\] and \[C{{H}_{3}}OH\] are x and y respectively, then which of the following relation is correct?
A)
\[x>y\]
done
clear
B)
\[x<y\]
done
clear
C)
\[x=y\]
done
clear
D)
\[x\ge y\]
done
clear
View Answer play_arrow
question_answer 75) The state of hybridisation of \[Cl\] atom in \[ClO_{2}^{-}\]is
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}}d\]
done
clear
D)
\[s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 76) The maximum number of electrons in an , subshell is given by the expression
A)
\[2{{n}^{2}}\]
done
clear
B)
\[4l+1\]
done
clear
C)
\[4l+2\]
done
clear
D)
\[4l-2\]
done
clear
View Answer play_arrow
question_answer 77) Which one of the silicon components does not exist?
A)
\[{{[Si{{F}_{6}}]}^{2-}}\]
done
clear
B)
\[Si{{(OH)}_{4}}\]
done
clear
C)
\[{{[SiC{{l}_{6}}]}^{2-}}\]
done
clear
D)
\[SiC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 78) The oxidation number of cobalt in \[K[Co{{(CO)}_{4}}]\] is
A)
+1
done
clear
B)
+2
done
clear
C)
- 1
done
clear
D)
- 3
done
clear
View Answer play_arrow
question_answer 79) Which one of the following complexes is paramagnetic in nature?
A)
\[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
B)
\[{{(Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 80) Constantan is an alloy of
A)
Cu, Sn
done
clear
B)
Cu, Zn
done
clear
C)
Cu, Ni
done
clear
D)
Cu, Mn
done
clear
View Answer play_arrow
question_answer 81) Electrolytic reduction method is used in extraction of
A)
highly electronegative elements
done
clear
B)
elements having high electron affinity
done
clear
C)
highly electropositive elements
done
clear
D)
transition elements
done
clear
View Answer play_arrow
question_answer 82) The undecomposed AgBr in black and white photography is dissolved by hypo solution due to formation of the complex.
A)
\[A{{g}_{2}}S\]
done
clear
B)
\[{{[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
C)
\[{{[Ag{{(S)}_{2}}]}^{3-}}\]
done
clear
D)
\[{{[Ag{{(S{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 83) Which of the following is the correct combination of oxidation states for group 17 elements?
A)
+ 6, + 4, + 2, -2
done
clear
B)
+ 7, + 5, + 3, + 1, - 1
done
clear
C)
+7, + 6, + 5, + 3, +2, -2
done
clear
D)
+ 7, + 5, + 4, + 3, + 2, - 2
done
clear
View Answer play_arrow
question_answer 84) The increasing electron releasing tendencies of Cu, Ag, Fe and Zn are in the order.
A)
Ag, Cu, Fe, Zn
done
clear
B)
Cu, Ag, Fe, Zn
done
clear
C)
Zn, Cu, Fe, Ag
done
clear
D)
Fe, Zn, Cu, Ag
done
clear
View Answer play_arrow
question_answer 85) The oxidation state of potassium in \[K{{O}_{2}}\] is
A)
+2
done
clear
B)
+ 4
done
clear
C)
+1
done
clear
D)
- 1
done
clear
View Answer play_arrow
question_answer 86) Which one of the following is Nesslefs reagent?
A)
\[{{K}_{2}}Hg{{l}_{4}}\]
done
clear
B)
\[{{K}_{2}}Hg{{l}_{4}}+KOH\]
done
clear
C)
\[{{K}_{2}}Hg{{l}_{2}}+KOH\]
done
clear
D)
\[KHg{{l}_{3}}+KOH\]
done
clear
View Answer play_arrow
question_answer 87) The presence of primary alcoholic group in glucose can be confirmed by
A)
oxidation of glucose with nitric acid
done
clear
B)
acetylation of glucose with acetic anhydride
done
clear
C)
oxidation of glucose with mild oxidizing etgent
done
clear
D)
prolonged heating of glucose with HI
done
clear
View Answer play_arrow
question_answer 88) The electronegativity of elements helps in predicting
A)
strength of the element
done
clear
B)
polarity of the molecule
done
clear
C)
size of the molecule
done
clear
D)
valency of the element
done
clear
View Answer play_arrow
question_answer 89) Which one of the following carbohydrate gives equimolar mixture of D-(+)-glucose and D-(-)-fructose on hydrolysis?
A)
Maltose
done
clear
B)
Lactose
done
clear
C)
Amylose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 90) The correct order of hybridisation of the central atom in the following species\[N{{H}_{3}},{{[PtC{{l}_{4}}]}^{2-}},PC{{l}_{5}}{{[BC{{l}_{4}}]}^{-}}\]
A)
\[s{{p}^{3}},ds{{p}^{2}},ds{{p}^{3}},s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}},ds{{p}^{2}},s{{p}^{3}},s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}},ds{{p}^{2}},ds{{p}^{3}},s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}},s{{p}^{2}},ds{{p}^{3}},s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 91) Carbylamine test is given by
A)
primary amines
done
clear
B)
secondary amines
done
clear
C)
test amines
done
clear
D)
quaternary amines
done
clear
View Answer play_arrow
question_answer 92) The product of the chemical reaction,\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-N{{H}_{2}}+B{{r}_{2}}+4NaOH\xrightarrow{{}}?\] will be
A)
\[C{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}-Br\]
done
clear
C)
\[C{{H}_{3}}OBr\]
done
clear
D)
\[C{{H}_{3}}-N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 93) Silver mirror test is given by
A)
amines
done
clear
B)
alcohols
done
clear
C)
carboxylic acids
done
clear
D)
aldehydes
done
clear
View Answer play_arrow
question_answer 94) In the chemical reaction,\[A\xrightarrow{{{O}_{3}}}B\xrightarrow{Zn+{{H}_{2}}O}\] propane-2-one + methanol. The compound A is
A)
2-methylbutene
done
clear
B)
but-1-ene
done
clear
C)
but-2-ene
done
clear
D)
1,3-butadiene
done
clear
View Answer play_arrow
question_answer 95) Tick the correct statement.
A)
Mixture of NaCI and \[N{{H}_{4}}Cl\] can be separated by sublimation
done
clear
B)
Mixture of camphor and \[N{{H}_{4}}Cl\] can be separated by sublimation
done
clear
C)
Mixture of \[NaCl\] and camphor can be separated by sublimation
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 96) Maximum number of optical isomers of 2, 3-dihydroxypentane are
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 97) Tick the statement that is not true about artificial sweetness.
A)
Aspartame is produced from phenylalanine
done
clear
B)
Alitame is used as common food sweetener
done
clear
C)
Sucrolose is stable at cooking temperature
done
clear
D)
o-sulphobenzimide does not provide calories to the human body
done
clear
View Answer play_arrow
question_answer 98) Tick the statement which is not true about nucleic acids?
A)
The hydrolysis of DNA produces \[\beta \]-D-2- deoxyribose
done
clear
B)
The hydrolysis of RNA produces thymine
done
clear
C)
The molecular mass of a nucleotide is greater than a nucleoside
done
clear
D)
Nucleotides are joined together by phosphodiester linkage to for dinucleotide
done
clear
View Answer play_arrow
question_answer 99) The chemical reaction,\[R-C{{H}_{2}}COOH\,\,\xrightarrow[(ii)\,\,{{H}_{2}}O]{(i)\,{{X}_{2}}/redphosphorus}R\]\[-\underset{\begin{smallmatrix} | \\ X \end{smallmatrix}}{\mathop{C}}\,H-COOH\]is known as
A)
Hunsdiecker reaction
done
clear
B)
Vilsmeier reaction
done
clear
C)
Finkelstein reaction
done
clear
D)
Hell-Volhard-Zelinsky reaction
done
clear
View Answer play_arrow
question_answer 100) Which one of the following organic compounds has highest boiling point?
A)
Pentan-1-al
done
clear
B)
Pentan-1-ol
done
clear
C)
n-pentane
done
clear
D)
Ethoxyethane
done
clear
View Answer play_arrow
question_answer 101) Adjacent nucleotides are joined by a
A)
covalent bond
done
clear
B)
phosphodiester bond
done
clear
C)
ionic bond
done
clear
D)
peptide bond
done
clear
View Answer play_arrow
question_answer 102) Axenic culture refers to
A)
culture lacking agar-agar
done
clear
B)
culture of cell
done
clear
C)
culture without contamination
done
clear
D)
culture of pollen
done
clear
View Answer play_arrow
question_answer 103) Bacteria protect themselves from viruses by fragmenting viral DNA upon entry with
A)
methylase
done
clear
B)
endonuclease
done
clear
C)
ligases
done
clear
D)
exonucleases
done
clear
View Answer play_arrow
question_answer 104) Genetic engineered male sterile crop plants may be produced by inserting
A)
Bt toxin gene
done
clear
B)
barnase gene
done
clear
C)
lectin gene
done
clear
D)
chitinase gene
done
clear
View Answer play_arrow
question_answer 105) The distribution of species diversity on earth may be best described as
A)
it is uniformity distributed
done
clear
B)
it is highest in tropics
done
clear
C)
it is highest in polar regions
done
clear
D)
it is highest in Southern hemisphere as lowest in Northern hemisphere
done
clear
View Answer play_arrow
question_answer 106) The correct statement is
A)
In a population, number of birth is different from birth rate.
done
clear
B)
A sigmoid growth curve is depiction of exponential growth.
done
clear
C)
In a logistic growth curve the asymptote is beyond the carrying capacity.
done
clear
D)
r is equal to the difference between number of births and number of deaths in a population.
done
clear
View Answer play_arrow
question_answer 107) The adaptation in an organism is meant for
A)
optimum primary production
done
clear
B)
optimum life span
done
clear
C)
optimum mobility
done
clear
D)
optimum survival and reproduction
done
clear
View Answer play_arrow
question_answer 108) Verhulst-PearP is associated with the equation
A)
\[\frac{dN}{dt}=rN\left( \frac{K-N}{K} \right)\]
done
clear
B)
\[\frac{dN}{dt}=tN\left( \frac{K-N}{K} \right)\]
done
clear
C)
\[\frac{dN}{dt}=rN\left( \frac{K-N}{N} \right)\]
done
clear
D)
\[\frac{dN}{dt}=tN\left( \frac{K-N}{N} \right)\]
done
clear
View Answer play_arrow
question_answer 109) The organism which can tolerate and thrive in a wide temperature range are known as
A)
eurythermal
done
clear
B)
isothermal
done
clear
C)
homothermal
done
clear
D)
stenothermal
done
clear
View Answer play_arrow
question_answer 110) A decline in population size will be in the stimulation
A)
nataiity < mortality
done
clear
B)
mortality < natality
done
clear
C)
immigration < emigration
done
clear
D)
emigration < immigration
done
clear
View Answer play_arrow
question_answer 111) The scientist widely regarded as the triple crown of biology X is
A)
RH Whittaker
done
clear
B)
Carolus Linnaeus
done
clear
C)
Ernst Mayr
done
clear
D)
Zimmermann
done
clear
View Answer play_arrow
question_answer 112) Rajaji National Park is situated in
A)
Tamil Nadu
done
clear
B)
Uttarakhand
done
clear
C)
Asom
done
clear
D)
Karnataka
done
clear
View Answer play_arrow
question_answer 113) Morels and buffles groups of fungi are classified under
A)
Phycomycetes
done
clear
B)
Deuteromycetes
done
clear
C)
Basidiomycetes
done
clear
D)
Ascomycetes
done
clear
View Answer play_arrow
question_answer 114) Bacterial viruses usually have
A)
single stranded RNA
done
clear
B)
double stranded RNA
done
clear
C)
single stranded DNA
done
clear
D)
double stranded DNA
done
clear
View Answer play_arrow
question_answer 115) No vessels are found in the wood of
A)
pine
done
clear
B)
Eucalyptus
done
clear
C)
teak
done
clear
D)
shesham
done
clear
View Answer play_arrow
question_answer 116) The increase in length of petiole results from the division of
A)
apical meristems
done
clear
B)
lateral meristems
done
clear
C)
intercalary meristems
done
clear
D)
phelogen
done
clear
View Answer play_arrow
question_answer 117) Oval, spherical or polygonal cells, thickening at the corners due to the deposition of cellulose, hemicellulose and pectin, often containing chloroplasts and having or not having intercellular spaces are called
A)
parenchyma
done
clear
B)
chlorenchyma
done
clear
C)
sclerenchyma
done
clear
D)
collenchyma
done
clear
View Answer play_arrow
question_answer 118) Monoadelphous condition of stamens is found in
A)
pea
done
clear
B)
China rose
done
clear
C)
Citrus
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 119) Floral formula of family-Fabaceae is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 120) Leaf tendrils are found in
A)
peas
done
clear
B)
cucumber
done
clear
C)
grape vines
done
clear
D)
All of theses
done
clear
View Answer play_arrow
question_answer 121) Smallest unit in the plant cell wall is
A)
micelle
done
clear
B)
microfibril
done
clear
C)
fibril
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 122) An example of mitogen is
A)
cytokinin
done
clear
B)
glucose
done
clear
C)
glycerol
done
clear
D)
fructose
done
clear
View Answer play_arrow
question_answer 123) Omnis cellula e cellula was stated by
A)
Schwann
done
clear
B)
Schleiden
done
clear
C)
Purkinje
done
clear
D)
Virchow
done
clear
View Answer play_arrow
question_answer 124) Cellulose is the polymer of
A)
\[\alpha \]-D glucose
done
clear
B)
\[\beta \]-D glucose
done
clear
C)
\[\alpha \]-D fructose
done
clear
D)
\[\beta \]-D fructose
done
clear
View Answer play_arrow
question_answer 125) The cell wall material present only in bacteria and blue-green algae is
A)
pectin
done
clear
B)
cellulose
done
clear
C)
chitin
done
clear
D)
muramic acids
done
clear
View Answer play_arrow
question_answer 126) A example of CAM plant is
A)
black night shade (Solanum nigrum)
done
clear
B)
lemon grass (Cymbopagon flexuosus)
done
clear
C)
sugarbeet (Beta vulgaris)
done
clear
D)
snake plant (Sanseviera thfasciata)
done
clear
View Answer play_arrow
question_answer 127) Abscisic acid cause
A)
faster leaf fall
done
clear
B)
dormancy of buds and seeds
done
clear
C)
retardation of growth
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 128) The characteristics like leaf expansion, breaking dormancy, promoting germination and flowering are associated with
A)
auxins
done
clear
B)
gibberellins
done
clear
C)
cytokinins
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 129) Die-back disease is caused by the deficiency of
A)
zinc
done
clear
B)
copper
done
clear
C)
manganese
done
clear
D)
boron
done
clear
View Answer play_arrow
question_answer 130) A natural growth regulator is
A)
2, 4-D.
done
clear
B)
benzaldehyde
done
clear
C)
naphthalene acetic acid
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 131) The enzyme nitrogenase is a
A)
Cu-Fe protein
done
clear
B)
Ni-Fe protein
done
clear
C)
Mo-Fe protein
done
clear
D)
Ni-Cu protein
done
clear
View Answer play_arrow
question_answer 132) The element related with nitrogen metabolism is
A)
manganese
done
clear
B)
magnesium
done
clear
C)
zinc
done
clear
D)
molybdenum
done
clear
View Answer play_arrow
question_answer 133) 6-(4-hydroxy-3 methyl-trans-2-butenylamine) purine is also called as
A)
methyl jasmonate
done
clear
B)
zeatin
done
clear
C)
brassinoide
done
clear
D)
triacontanol
done
clear
View Answer play_arrow
question_answer 134) The isobilateral type of microspores arrangement in tetrad is present in
A)
Solanum nigrum
done
clear
B)
Zea mays
done
clear
C)
Cassia fistula
done
clear
D)
Vigna radiate
done
clear
View Answer play_arrow
question_answer 135) The fibrous bands develop from cells of the another wall layer known as
A)
epidermis
done
clear
B)
endothecium
done
clear
C)
middle layers
done
clear
D)
tapetum
done
clear
View Answer play_arrow
question_answer 136) Chalazogamy is shown by
A)
Petunia
done
clear
B)
Cucurbita
done
clear
C)
Pistacia
done
clear
D)
Casuarina
done
clear
View Answer play_arrow
question_answer 137) Anthesis is a phenomenon which refers to
A)
stigma receptivity
done
clear
B)
dehiscence of anthers
done
clear
C)
viability of pollen
done
clear
D)
opening of flower bud
done
clear
View Answer play_arrow
question_answer 138) Downs syndrome is characterised by
A)
19trisomy
done
clear
B)
21 trisomy
done
clear
C)
one X-chromosome
done
clear
D)
two X and one Y-chromosome
done
clear
View Answer play_arrow
question_answer 139) The enzyme used to join the fragments of DNA during the process of replication is
A)
DNA polymerase
done
clear
B)
DNA ligase
done
clear
C)
endonuclease
done
clear
D)
helicase
done
clear
View Answer play_arrow
question_answer 140) Semi-conservative replication of DNA was first demonstrated in
A)
Eschenchia coli
done
clear
B)
Streptococcus pneumonia
done
clear
C)
Drosophila melanogaster
done
clear
D)
Salmonella typhimurium
done
clear
View Answer play_arrow
question_answer 141) The transfer of genetic material from the bacterium to another via viruses is called
A)
transformation
done
clear
B)
conjugation
done
clear
C)
recombination
done
clear
D)
transduction
done
clear
View Answer play_arrow
question_answer 142) During the experiments, Mendel called genes by the term
A)
traits
done
clear
B)
characters
done
clear
C)
factors
done
clear
D)
qualities
done
clear
View Answer play_arrow
question_answer 143) If a genotype consist of different types of alleles, it is called.
A)
homozygous
done
clear
B)
heterozygous
done
clear
C)
monoallelic
done
clear
D)
uniallelic
done
clear
View Answer play_arrow
question_answer 144) The alternative form of gene is called
A)
dominant character
done
clear
B)
recessive character
done
clear
C)
alternative genes
done
clear
D)
allele
done
clear
View Answer play_arrow
question_answer 145) The experimental material in Mendels experiment was
A)
Pisum sativum
done
clear
B)
Oryza sativa
done
clear
C)
Mirabilis jalapa
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 146) Beer has an alcoholic content of
A)
3-6%
done
clear
B)
9-12%
done
clear
C)
40%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 147) Triticale is an example of
A)
auto polyploidy
done
clear
B)
allopolyploidy
done
clear
C)
aneuploidy
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 148) The common bread wheat has
A)
14 chromosome
done
clear
B)
21 chromosome
done
clear
C)
28 chromosome
done
clear
D)
42 chromosome
done
clear
View Answer play_arrow
question_answer 149) In tissue culture, shoot formation in callus can be induced by
A)
IAA
done
clear
B)
ABA
done
clear
C)
GAB
done
clear
D)
kinetin
done
clear
View Answer play_arrow
question_answer 150) Restriction endonucleases are
A)
used for in vitro DNA synthesis
done
clear
B)
synthesised by bacteria as part of their defense mechanism
done
clear
C)
present in mammalian cells for degradation of DNA when the cells dies
done
clear
D)
used in genetic engineering for ligating two DNA molecules
done
clear
View Answer play_arrow
question_answer 151) Which of the following is a mismatch pair?
A)
Scales - Reptiiia
done
clear
B)
Combplates - Mollusca
done
clear
C)
Choanocytes - Porifera
done
clear
D)
Parapodia - Annelida
done
clear
View Answer play_arrow
question_answer 152) Vermiform appendix arises from
A)
caecum
done
clear
B)
colon
done
clear
C)
rectum
done
clear
D)
ileum
done
clear
View Answer play_arrow
question_answer 153) Which of the following sets of conditions promotes the dissociation of oxygen from haemoglobin?
A)
Low \[p{{O}_{2}}\], high \[pC{{O}_{2}}\], high \[{{H}^{+}}\]
done
clear
B)
High \[p{{O}_{2}}\], high \[pC{{O}_{2}}\], low \[{{H}^{+}}\]
done
clear
C)
High \[pC{{O}_{2}}\], low \[pC{{O}_{2}}\], low \[{{H}^{+}}\]
done
clear
D)
Low \[p{{O}_{2}}\], low \[pC{{O}_{2}}\], low \[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 154) Choose the correct statement.
A)
Stroke volume multiplied by cardial output gives the heart rate.
done
clear
B)
Heart rate multiplied by cardiac output gives the stroke volume.
done
clear
C)
Cardiac output divided by heart rate gives the stroke volume.
done
clear
D)
Stroke volume divided by heart rate gives the cardiac output.
done
clear
View Answer play_arrow
question_answer 155) The juxta glomerular apparatus is a special region formed by close placement and cellular modification in
A)
proximai convoluted tubule and distal convoluted tubule
done
clear
B)
loop of Henie and collecting duct
done
clear
C)
afferent arteriole and distal convoluted tubule
done
clear
D)
afferent arteriole and proximai convoluted tubule
done
clear
View Answer play_arrow
question_answer 156) In humans dosage compensation
A)
brings about equality in X-coded gene product
done
clear
B)
brings about equality in Y-coded gene products
done
clear
C)
brings about determination of sex
done
clear
D)
is not involved in any of above
done
clear
View Answer play_arrow
question_answer 157) Foot prints, trails, tracks and tunnels of various organisation made in mud are rapidly filled in with sand and covered by sediments. This is an example of which of the following type of fossil?
A)
Pertified fossil
done
clear
B)
Impressions
done
clear
C)
Imprints
done
clear
D)
Coprolites
done
clear
View Answer play_arrow
question_answer 158) Which of the following belongs to class-Polyplacophora?
A)
Chiton
done
clear
B)
Patella
done
clear
C)
Pila
done
clear
D)
Murex
done
clear
View Answer play_arrow
question_answer 159) Proportion of colour blind children when normal man marries to carrier woman is
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 160) Which of the following cells secretes histamine?
A)
Plasma cells,
done
clear
B)
Mast cells
done
clear
C)
Monocytes
done
clear
D)
NK-cells
done
clear
View Answer play_arrow
question_answer 161) The retention of juvenile morphological traits in the sexually mature adult
A)
mimicry
done
clear
B)
neoteny
done
clear
C)
vivipary
done
clear
D)
hybrid vigour
done
clear
View Answer play_arrow
question_answer 162) Ionosphere is between
A)
stratosphere and mesosphere
done
clear
B)
mesosphere and thermosphere
done
clear
C)
troposphere and stratosphere
done
clear
D)
troposphere and thermosphere
done
clear
View Answer play_arrow
question_answer 163) The following hormone is not a steroid
A)
testosterone
done
clear
B)
progesterone
done
clear
C)
corticosteroids
done
clear
D)
adrenocorticotrophic hormone
done
clear
View Answer play_arrow
question_answer 164) Tetraiodothyronine refers to
A)
13
done
clear
B)
Thyroxine
done
clear
C)
TSH
done
clear
D)
TRH
done
clear
View Answer play_arrow
question_answer 165) The pneumotaxic centre and rhythm centre are respectively present in
A)
pons and medulla oblongata
done
clear
B)
corpus callosum and pons
done
clear
C)
medulla oblongata and hypothalamus
done
clear
D)
diencephalon and pons
done
clear
View Answer play_arrow
question_answer 166) Which of the following bones does not articulate with any other bone?
A)
Humerus
done
clear
B)
Malleus
done
clear
C)
Phalanges
done
clear
D)
Hyoid
done
clear
View Answer play_arrow
question_answer 167) Which of the following is most appropriate regarding kidney function regulation? Stimulation of
A)
Renin-angiotensin mechanism decreases the Glomerular Filtration Rate (GFR) while atrial natriuretic factor increase GFR
done
clear
B)
Renin-angiotensin mechanism increase GFR while atrial natriuretic factor decreases GFR
done
clear
C)
Renin-angiotensin mechanism and atrial natriuretic factor both increase the GFR
done
clear
D)
Renin-angiotensin mechanism and atrial natriuretic factor both decrease the GFR
done
clear
View Answer play_arrow
question_answer 168) The primary neurotransmitter at the neuromuscular junction is
A)
dopamine
done
clear
B)
adrenaline
done
clear
C)
acetylcholine
done
clear
D)
acetaldehyde
done
clear
View Answer play_arrow
question_answer 169) The optimum pH for pepsin is
A)
11
done
clear
B)
5-6
done
clear
C)
1.6-2.4
done
clear
D)
4-7
done
clear
View Answer play_arrow
question_answer 170) The red jungle fowl is
A)
Gallus gallus
done
clear
B)
Ravo cristatus
done
clear
C)
Choriotis nigriceps
done
clear
D)
Raja hansa
done
clear
View Answer play_arrow
question_answer 171) The snow leopard is
A)
Panthera pardus
done
clear
B)
Panthera uncial
done
clear
C)
Panthera tigris
done
clear
D)
Hylobates hoolock
done
clear
View Answer play_arrow
question_answer 172) The molting hormone ecdysone belongs to the category of
A)
amines
done
clear
B)
long chain peptides
done
clear
C)
steroids
done
clear
D)
fatty acids
done
clear
View Answer play_arrow
question_answer 173) Secretion of which of the following hormones is not pituitary dependant?
A)
Triiodothyronine
done
clear
B)
Testosterone
done
clear
C)
Glucocorticoids
done
clear
D)
Parathyroid hormone
done
clear
View Answer play_arrow
question_answer 174) Sickle-cell anaemia results due to mutation caused by
A)
substitution
done
clear
B)
insertion
done
clear
C)
deletion
done
clear
D)
duplication
done
clear
View Answer play_arrow
question_answer 175) Which of the following DNA polymerase of prokaryotes have both 3-5 and 5-3 exonuclease activity?
A)
DNA polymerase-II
done
clear
B)
DNA polymerase-I
done
clear
C)
DNA polymerase-IV
done
clear
D)
DNA polymerase-III
done
clear
View Answer play_arrow
question_answer 176) Which of the following is a disaccharide?
A)
Glucose
done
clear
B)
Lactose
done
clear
C)
Starch
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 177) Enzyme not present in pancreatic juice is
A)
amylase
done
clear
B)
chymotrypsinogen
done
clear
C)
lipase
done
clear
D)
enterokinase
done
clear
View Answer play_arrow
question_answer 178) Trisomy 18 is
A)
Edwards syndrome
done
clear
B)
Pataus syndrome
done
clear
C)
Turners syndrome
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 179) The hormones that are produced in women only during pregnancy
A)
oestrogens, human chorionic gonadotropin, human placental lactogen
done
clear
B)
oestrogen, progesteron, oxytocin
done
clear
C)
human placental lactogen, human chorionic gonadotropin, relaxin
done
clear
D)
human placental lactogen, human chorionic gonadotropin, thyroxine
done
clear
View Answer play_arrow
question_answer 180) The juvenile hormone promoting the nymphal characteristics of insects, is produced by
A)
prothoracic glands
done
clear
B)
corpus cardiacum
done
clear
C)
corpus allatum
done
clear
D)
corpus albicans
done
clear
View Answer play_arrow
question_answer 181) Which of the following antibody is pentamer?
A)
IgA
done
clear
B)
IgD
done
clear
C)
IgE
done
clear
D)
IgM
done
clear
View Answer play_arrow
question_answer 182) The cercarial stage of a liver-fluke is produced by
A)
sexual multiplication
done
clear
B)
asexual multiplication
done
clear
C)
binary fission
done
clear
D)
parthenogenesis .
done
clear
View Answer play_arrow
question_answer 183) Which one of the following is an actively swimming, free-living and non-feeding stage of the liver-fluke?
A)
Sporocyst
done
clear
B)
Redia
done
clear
C)
Cercaria
done
clear
D)
Metacercaria
done
clear
View Answer play_arrow
question_answer 184) Which one of the following cannot be attributed to glycogen?
A)
Homopolysaccharide
done
clear
B)
Heteropolysaccharide
done
clear
C)
Branched chain molecule
done
clear
D)
Stored in liver and muscle
done
clear
View Answer play_arrow
question_answer 185) With respect to its body mass which of the following will have highest metabolic rate?
A)
Rat
done
clear
B)
Rabbit
done
clear
C)
Horse
done
clear
D)
Elephant
done
clear
View Answer play_arrow
question_answer 186) Which one of the following vitamin is not fat soluble?
A)
A
done
clear
B)
B
done
clear
C)
D
done
clear
D)
E
done
clear
View Answer play_arrow
question_answer 187) The deficiency of which of the following vitamin will cause xerophthalmia?
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 188) At what speed the mitochondria can be separated out by differential centrifugation?
A)
200 xg
done
clear
B)
500 xg
done
clear
C)
800 xg
done
clear
D)
8000 xg
done
clear
View Answer play_arrow
question_answer 189) The amoeboid movement results from
A)
interactions among actin, myosin and ATP, etc
done
clear
B)
coordinated beats of cilia
done
clear
C)
whip-like action of flagella
done
clear
D)
action by the mitotic spindle, similar so, what happens during mitosis and meiosis
done
clear
View Answer play_arrow
question_answer 190) A donor having blood group AB can safely donate red blood cells to recipients having blood group type
A)
A and AB
done
clear
B)
B and AB
done
clear
C)
Only AB
done
clear
D)
Only 0
done
clear
View Answer play_arrow
question_answer 191) Which of the following is the most appropriate in normal circumstances?
A)
During inspiration the intrapulmonary pressure is less than the atmospheric pressure
done
clear
B)
During expiration, the intrapulmonary pressure is less than the atmospheric pressure
done
clear
C)
During inspiration, the intrapulmonary pressure is more than the atmospheric pressure
done
clear
D)
During expiration the intrapulmonary pressure is equal to the atmospheric pressure
done
clear
View Answer play_arrow
question_answer 192) Which of the following best explains the difference between an epitope and an antigen?
A)
An epitope is any foreign substance, an antigen is a foreign protein
done
clear
B)
An epitope is the part of an antigen where an antibody or lymphocyte receptor binds
done
clear
C)
An antigen is the part of an epitope where on antibody or lymphocyte receptor binds
done
clear
D)
Antigens are recognised by B-cells and antibodies; epitopes are recognised by T-cells
done
clear
View Answer play_arrow
question_answer 193) Which of the following is correct regarding RNA processing?
A)
In capping, methyl guanosine triphosphate is added to 3 and of hn RNA
done
clear
B)
RNA polymerase-1 transcribes? RNA in eukaryotes
done
clear
C)
In tailing, adenylate residues are added at 3end of hn-RNA
done
clear
D)
Three types of RNA polymerase catalyse the transcription of these different types of RNAs in most bacteria
done
clear
View Answer play_arrow
question_answer 194) Splicing is the process where
A)
exons are removed from growing ?RNA strand
done
clear
B)
introns are removed from growing polypeptide chain
done
clear
C)
introns are removed from heterogenous nuclear RNA
done
clear
D)
exons are removed from mRna
done
clear
View Answer play_arrow
question_answer 195) Which plant drug pair is incorrect?
A)
Papaver somniferum - Morphine
done
clear
B)
Cannabis sativa - Marijuana
done
clear
C)
Erythroxylum coca - Cocine
done
clear
D)
Atropa belladona - Hashish
done
clear
View Answer play_arrow
question_answer 196) Which of the following statement is incorrect?
A)
Oceans contain about 71 % of total global carbon.
done
clear
B)
Atmosphere contain about 71% of total global carbon.
done
clear
C)
Carbon constitute about 49% of the dry weight of organism,
done
clear
D)
Approximately \[4\times {{10}^{13}}\] kg of carbon is fixed in the biosphere through photosynthesis annually.
done
clear
View Answer play_arrow
question_answer 197) The Verhulst-Pearl logistic growth is described using the equation \[\frac{dN}{dt}=rN\left( \frac{K-N}{K} \right)\], in this K stands tor.
A)
temperature in degree Kelvin
done
clear
B)
intrinsic rate of natural increase
done
clear
C)
carrying capacity
done
clear
D)
population density
done
clear
View Answer play_arrow
question_answer 198) Partial pressures of oxygen and carbon dioxide in healthy human lung alveoli are respectively, nearest to
A)
104 and 40 mm of Hg
done
clear
B)
90 and 20 mm of Hg
done
clear
C)
40 and 46 mm of Hg
done
clear
D)
159 and 0.3 mm of Hg
done
clear
View Answer play_arrow
question_answer 199) Which of the following exhibits metagenesis?
A)
Hydra
done
clear
B)
Adamsia
done
clear
C)
Aurelia
done
clear
D)
Obelia
done
clear
View Answer play_arrow
question_answer 200) Pick the odd one out.
A)
Chelone, Calotes, Naja
done
clear
B)
Pavo, Psittacula, Ornithorhynchus
done
clear
C)
Cams, Felis, Rattus
done
clear
D)
Bufo, Rana, Hyla
done
clear
View Answer play_arrow