question_answer 1) Which one of the following is the dimensionless quantity?
A)
Plancks constant
done
clear
B)
Dielectric constant
done
clear
C)
Solid angle
done
clear
D)
Strain
done
clear
View Answer play_arrow
question_answer 2) A car travels half of the distance with constant velocity of 30 km/h and another half with a constant velocity of 50 km/h along a straight line. The average velocity of the car in km/h is:
A)
38.7
done
clear
B)
42
done
clear
C)
37.5
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 3) A train of length ISO m is going towards north direction at the speed of 10 m/s. A parrot flies at a speed of 5 m/s towards south direction parallel to the railway track then the time taken by the parrot to cross the train is equal to:
A)
15 s
done
clear
B)
10 s
done
clear
C)
12 s
done
clear
D)
8 s
done
clear
View Answer play_arrow
question_answer 4) The position vector of a particle is determined by the expression\[\vec{r}=3{{t}^{2}}\hat{i}+4{{t}^{2}}\hat{j}+7\hat{k}\]. The distance traversed in first 10 s is :
A)
500 m
done
clear
B)
300 m
done
clear
C)
150 m
done
clear
D)
100 m
done
clear
View Answer play_arrow
question_answer 5) The horizontal range of a projectile is \[4\sqrt{3}\] times of its maximum height, the angle of projection will be :
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[{{40}^{o}}\]
done
clear
View Answer play_arrow
question_answer 6) A body is accelerated from rest by applying a force of 30 N. The momentum of the body after 2 s will be :
A)
60 kg m/s
done
clear
B)
120 kg m/s
done
clear
C)
67.5kg m/s
done
clear
D)
30 kg m/s
done
clear
View Answer play_arrow
question_answer 7) A car weighing 500 kg working against a resistance of 500 N, accelerates from rest to 20 m/s within 100 s. The work done by the engine of car will be : \[(g=10\,m/{{s}^{2}})\]
A)
\[1.05\times {{10}^{5}}J\]
done
clear
B)
\[1.0\times {{10}^{4}}J\]
done
clear
C)
\[15\times {{10}^{4}}J\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 8) An object A of mass 2 kg is moving with a velocity of 3 m/s and collides head-on with an object B of mass 1 kg moving in opposite direction with a velocity of 4 m/s. After collision both objects coalesce, so that they move with a common velocity equal to :
A)
3 m/s
done
clear
B)
2 m/s
done
clear
C)
1 m/s
done
clear
D)
\[\frac{2}{3}\,m/s\]
done
clear
View Answer play_arrow
question_answer 9) The radius of earth is about 6400 km and that of mars is about 3200 km. The mass of the earth is about 10 times the mass of mars. A object weighs 200 N on earths surface, then its weight on the surface of mars will be :
A)
80 N
done
clear
B)
40 N
done
clear
C)
8N
done
clear
D)
20 N
done
clear
View Answer play_arrow
question_answer 10) A body executing simple harmonic motion has a maximum acceleration equal to \[24\,m/{{s}^{2}}\]and maximum velocity equal to 16 m/s. The amplitude of SHM is :
A)
\[\frac{64}{9}m\]
done
clear
B)
\[\frac{1024}{9}m\]
done
clear
C)
\[\frac{3}{32}m\]
done
clear
D)
\[\frac{32}{9}m\]
done
clear
View Answer play_arrow
question_answer 11) Assuming earth to be a sphere of uniform density. What is the value of acceleration due to gravity at a point 100 km below the earths surface? (Given, \[R=6380\times {{10}^{3}}m\])
A)
\[3.6\,m/{{s}^{2}}\]
done
clear
B)
\[6.6\,m/{{s}^{2}}\]
done
clear
C)
\[7.66\,m/{{s}^{2}}\]
done
clear
D)
\[9.65\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) A bullet is tired from a gun with a speed of 1000 m/s in order to hit a target 100 m away. The height above the target the gun should be aimed will be : (The resistance of air is negligible and \[g=10\,m/{{s}^{2}}\])
A)
25 cm
done
clear
B)
12 cm
done
clear
C)
9 cm
done
clear
D)
5 cm
done
clear
View Answer play_arrow
question_answer 13) In a thermodynamic process, pressure of a fixed mass of a gas is changed in such a manner that the gas releases 20 J of heat and 8 J of work is done on the gas. If the initial internal energy of the gas was 30 J, the final internal energy will be:
A)
18 J
done
clear
B)
9 J
done
clear
C)
4.5 J
done
clear
D)
36 J
done
clear
View Answer play_arrow
question_answer 14) Two bodies are at temperatures \[{{27}^{o}}C\]and. The \[{{927}^{o}}C\] heat energy radiated by them will be in the ratio :
A)
\[1:256\]
done
clear
B)
\[1:64\]
done
clear
C)
\[1:4\]
done
clear
D)
\[1:16\]
done
clear
View Answer play_arrow
question_answer 15) The efficiency of a Carnot engine operating between reservoirs maintained at temperatures \[{{27}^{o}}C\] and \[={{123}^{o}}C\] is:
A)
0.75
done
clear
B)
0.4
done
clear
C)
0.25
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 16) At Rihand dam project water falls from a height of 210 m. Assuming whole of energy due to fall is converted into heat, the rise in temperature of water would be, (J = 4.2 J/cal):
A)
\[{{490}^{o}}C\]
done
clear
B)
\[{{49}^{o}}C\]
done
clear
C)
\[{{0.49}^{o}}C\]
done
clear
D)
\[{{4.9}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 17) At what temperature the speed of sound in air will become double of its value at \[{{27}^{o}}C\]?
A)
\[{{54}^{o}}C\]
done
clear
B)
\[{{627}^{o}}C\]
done
clear
C)
\[{{927}^{o}}C\]
done
clear
D)
\[{{327}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 18) The frequency of a tuning fork is 384/s and velocity of sound in air is 352 m/s. Find how far the sound has traversed while fork completes 36 vibrations.
A)
33 m
done
clear
B)
16.5 m
done
clear
C)
11 m
done
clear
D)
22 m
done
clear
View Answer play_arrow
question_answer 19) An air column pipe closed at one end and open at the other end, resonates with a tuning fork and when 45 cm, 99 cm are two other lengths in between these values, the wavelength of sound in air column is :
A)
36cm
done
clear
B)
54cm
done
clear
C)
108cm
done
clear
D)
180cm
done
clear
View Answer play_arrow
question_answer 20) The equation of a stationary wave is,\[y=5\,\sin \,\frac{\pi x}{3\,}\,\cos \,40\,\pi t\] where\[x\] and \[y\] are in cm and t in second. The separation between two consecutive nodes is :
A)
1.5cm
done
clear
B)
6.0cm
done
clear
C)
3cm
done
clear
D)
12cm
done
clear
View Answer play_arrow
question_answer 21) In a hydrogen atom, the electron revolves round the nucleus in an orbit of radius \[0.53\times {{10}^{-10}}m\]. The electric potential produced by the nucleus at the position of the electron is :
A)
17.2V
done
clear
B)
23.6V
done
clear
C)
13.6V
done
clear
D)
27.2V
done
clear
View Answer play_arrow
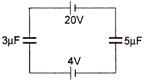
question_answer 22)
In the circuit shown in the given figure the potential difference across \[3\mu F\]capacitor is:
A)
16V
done
clear
B)
10V
done
clear
C)
4V
done
clear
D)
6V
done
clear
View Answer play_arrow
question_answer 23) A parallel plate capacitor has plates of area \[200\,c{{m}^{2}}\] and separation 0.05 cm. It has been charged to a potential difference of 300 V, the energy of the capacitor is :
A)
\[2.0\times {{10}^{-6}}J\]
done
clear
B)
\[2.4\times {{10}^{-5}}\]
done
clear
C)
\[0.8\times {{10}^{-5}}J\]
done
clear
D)
\[1.6\times {{10}^{-5}}J\]
done
clear
View Answer play_arrow
question_answer 24) If 2.2 kW power is transmitted through a \[10\,\,\Omega \]line at 22000 V, the power loss per second in the form of heat will be :
A)
100 W
done
clear
B)
10W
done
clear
C)
1 W
done
clear
D)
0. 1W
done
clear
View Answer play_arrow
question_answer 25) Two 1000 W heaters when connected in parallel across 220 V supply produce heat \[{{Q}_{p}}\] in time t. If they are connected in series across the same power supply the heat produced in the same time is \[{{Q}_{s}}\] then \[{{Q}_{P}}/{{Q}_{S}}\] will be :
A)
0.25
done
clear
B)
0.5
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 26) The resistance of a tungsten filament at \[{{150}^{o}}C\]is \[133\,\Omega \] What will be its resistance at \[{{500}^{o}}C\]? The temperature coefficient of resistance of tungsten is 0.0045.
A)
\[258\,\,\Omega \]
done
clear
B)
\[300\,\,\Omega \]
done
clear
C)
\[158\,\,\Omega \]
done
clear
D)
\[58\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 27) The resistance of the wire is R ohm. Its new resistance, if it is stretched n times to its original length, will be :
A)
\[nR\]
done
clear
B)
\[\frac{n}{2}R\]
done
clear
C)
\[n{{R}^{2}}\]
done
clear
D)
\[{{n}^{2}}R\]
done
clear
View Answer play_arrow
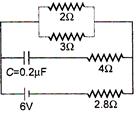
question_answer 28)
In the given figure the steady state current in \[2\,\,\Omega \] resistor is :
A)
zero
done
clear
B)
0.6 A
done
clear
C)
0.9 A
done
clear
D)
1.5A
done
clear
View Answer play_arrow
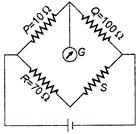
question_answer 29)
The Wheatstone bridge shown in figure is balanced. The resistance S is :
A)
\[0.7\,\,\Omega \]
done
clear
B)
\[70\,\,\Omega \]
done
clear
C)
\[7\,\,\Omega \]
done
clear
D)
\[700\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 30) If resistance of a galvanometer is \[6\,\,\Omega \] and it can measure a maximum current of 2 A, then the required shunt resistance to convert it into an ammeter reading upto 6 A will be :
A)
\[5\,\,\Omega \]
done
clear
B)
\[4\,\,\Omega \]
done
clear
C)
\[3\,\,\Omega \]
done
clear
D)
\[2\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 31) A driving current of 2 A for 6 min in a circuit is flowing then 1000 J of work is to be done. The en-if of the source in the circuit is :
A)
1.38V
done
clear
B)
1.68V
done
clear
C)
2.03V
done
clear
D)
3.10V
done
clear
View Answer play_arrow
question_answer 32) A charged particle is moving in uniform magnetic field in a circular path. The radius of circular path is R. When energy of particle is doubled, then new radius will be :
A)
\[\frac{R}{\sqrt{2}}\]
done
clear
B)
\[2R\]
done
clear
C)
\[\frac{R}{2}\]
done
clear
D)
\[\sqrt{2}R\]
done
clear
View Answer play_arrow
question_answer 33) Radius of First orbit of hydrogen is \[0.53\,\overset{o}{\mathop{A}}\,\]. The radius in fourth orbit is :
A)
\[8.48\,\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[2.12\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[4.24\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[0.193\,\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 34) The light of wavelength 5000 A is incident on a metal whose work function is 2eV, the maximum kinetic energy of the photoelectron will be :
A)
0.2eV
done
clear
B)
0.47eV
done
clear
C)
1.5eV
done
clear
D)
2eV
done
clear
View Answer play_arrow
question_answer 35) Slow neutrons can cause fission in :
A)
uranium-238
done
clear
B)
uranium-235
done
clear
C)
hydrogen
done
clear
D)
thorium
done
clear
View Answer play_arrow
question_answer 36) In a pure electric conductor the width of the forbidden band is :
A)
5 to 10eV
done
clear
B)
2.1eV
done
clear
C)
0.8eV
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 37) Magnetic permeability is maximum for :
A)
paramagnetic substances
done
clear
B)
ferromagnetic substances
done
clear
C)
diamagnetic substances
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 38) Magnetic field along the axis at a point distance d from a short bar magnet, is :
A)
\[\frac{{{\mu }_{0}}}{3N}\times \frac{M}{{{d}^{3}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{N}\times \frac{M}{{{d}^{3}}}\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{2\pi }\times \frac{M}{{{d}^{3}}}\]
done
clear
D)
\[\frac{{{\mu }_{0}}}{4\pi }\times \frac{M}{{{d}^{3}}}\]
done
clear
View Answer play_arrow
question_answer 39) A coil has an inductance of 0.03 H. The emf induced, when the current in the coil changes at a rate of 200 A/s, will be :
A)
15V
done
clear
B)
12V
done
clear
C)
9 C
done
clear
D)
6 V
done
clear
View Answer play_arrow
question_answer 40) The work function of a photometal is 6.626eV the threshold wavelength will be :
A)
\[1575\,\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[1868\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[2875\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[3921\,\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 41) A prism has a refracting angle of \[{{60}^{o}}\]. It produces a minimum deviation of \[{{30}^{o}}\], the angle of incidence is :
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{35}^{o}}\]
done
clear
D)
\[{{15}^{o}}\]
done
clear
View Answer play_arrow
question_answer 42) A convex lens forms a real image of an object for its two different positions on the screen. If height of the images in two cases are 8 cm and 2 cm, then the height of the object will be :
A)
2 cm
done
clear
B)
4 cm
done
clear
C)
8 cm
done
clear
D)
16 cm
done
clear
View Answer play_arrow
question_answer 43) If 1 g of hydrogen is converted into 0.993 g of helium in a thermonuclear reaction, then the energy released in this reaction will be:
A)
\[0.63\times {{10}^{10}}J\]
done
clear
B)
\[6.63\times {{10}^{14}}J\]
done
clear
C)
\[63\times {{10}^{10}}J\]
done
clear
D)
\[0.63\times {{10}^{7}}J\]
done
clear
View Answer play_arrow
question_answer 44) In junction diode, the holes are due to :
A)
missing electron
done
clear
B)
proton
done
clear
C)
neutron
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 45) The setting sun looks red because of:
A)
external reflection
done
clear
B)
internal reflection
done
clear
C)
scattering of light
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 46) An X-ray machine is operated at 40 kV. deduce the short wavelength bisect of continuous X-ray \[(h=6.63\times {{10}^{34}}\,Js,c=3\times {{10}^{8}}\,m/s,e=1.6\times {{10}^{-19}}C)\]
A)
\[0.31\,\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[0.62\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[1.62\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[1.31\,\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 47) Yellow sodium light has wavelength of \[5893\,\overset{o}{\mathop{A}}\,\] then its frequency will be :
A)
\[16.25\times {{10}^{20}}Hz\]
done
clear
B)
\[9.20\times {{10}^{19}}Hz\]
done
clear
C)
\[7.15\times {{10}^{15}}Hz\]
done
clear
D)
\[5.09\times {{10}^{14}}Hz\]
done
clear
View Answer play_arrow
question_answer 48) If an electron moves in a circular path of radium 15 cm in a magnetic Held of \[4\times {{10}^{-4}}T\], the speed of electron will be :
A)
\[5.87\times {{10}^{8}}m/s\]
done
clear
B)
\[3.01\times {{10}^{4}}m/s\]
done
clear
C)
\[2.03\times {{10}^{6}}m/s\]
done
clear
D)
\[1.06\times {{10}^{7}}m/s\]
done
clear
View Answer play_arrow
question_answer 49) The peak value of an alternating current frequency 50 Hz is 14.14 A. Find the rms value of current, and how much time will the current take in reaching from zero to maximum value?
A)
10 A, 0.005s
done
clear
B)
15 A, 0.05s
done
clear
C)
12 A, 0.05s
done
clear
D)
8 A, 0.01s
done
clear
View Answer play_arrow
question_answer 50) The half-life of radium is 1600 yr. What fraction of sample of radium will be disintegrated after 6400 yr?
A)
\[\frac{13}{16}\]
done
clear
B)
\[\frac{1}{16}\]
done
clear
C)
\[\frac{8}{17}\]
done
clear
D)
\[\frac{15}{16}\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following on hydrolysis, forms acetic acid?
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 52) Principle product (X) in the reaction \[C{{H}_{3}}CON{{H}_{2}}\xrightarrow[B{{r}_{2}}+NaOH]{}X\] is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}Br\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 53) The molecule with highest percentage of ionic character is:
A)
HF
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
HI
done
clear
View Answer play_arrow
question_answer 54) Oxidation number of P in \[{{H}_{3}}P{{O}_{2}}\] is:
A)
+ 5
done
clear
B)
+ 3
done
clear
C)
+ 2
done
clear
D)
+ 1
done
clear
View Answer play_arrow
question_answer 55) Catalyst used in Friedel-Craffs reaction is:
A)
anhydrous aluminium chloride
done
clear
B)
sodium
done
clear
C)
ferric chloride
done
clear
D)
zinc oxide
done
clear
View Answer play_arrow
question_answer 56) A compound with empirical formula \[C{{H}_{2}}O\] has a vapour density of 30. Its molecular formula is:
A)
\[{{C}_{3}}{{H}_{6}}{{O}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
C)
\[C{{H}_{2}}O\]
done
clear
D)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following is optically active?
A)
Tartaric acid
done
clear
B)
Glycerol
done
clear
C)
Ethylene glycol
done
clear
D)
Oxalic acid
done
clear
View Answer play_arrow
question_answer 58) Aldehydes and ketones can be distinguished by:
A)
Lucas test
done
clear
B)
Tollens reagent
done
clear
C)
Molishtest
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 59) The acetic acid reacts with \[NaOH\] to give:
A)
\[HCOONa\]
done
clear
B)
\[C{{H}_{3}}COONa\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 60) Toluene on oxidation with chromyl chloride gives:
A)
benzoic acid
done
clear
B)
benzaldehyde
done
clear
C)
benzoylchloride
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 61) Le-Chatelier principle is applicable only to a:
A)
system in equilibrium
done
clear
B)
irreversible reaction
done
clear
C)
homogeneous reaction
done
clear
D)
heterogeneous reaction
done
clear
View Answer play_arrow
question_answer 62) Gibbs free energy G is defined as :
A)
\[G=H-TS\]
done
clear
B)
\[G=H+TS\]
done
clear
C)
\[G=E+TS\]
done
clear
D)
\[G=E-TS\]
done
clear
View Answer play_arrow
question_answer 63) Sweetest sugar is:
A)
fructose
done
clear
B)
maltose
done
clear
C)
glucose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 64) Hydrolysis of sucrose is called :
A)
inversion
done
clear
B)
esterification
done
clear
C)
saponification
done
clear
D)
hydration
done
clear
View Answer play_arrow
question_answer 65) Which of the following enzyme increases the pulse rate and blood pressure?
A)
Estrone
done
clear
B)
Cortisone
done
clear
C)
Adrenaline
done
clear
D)
Thyroxine
done
clear
View Answer play_arrow
question_answer 66) Which ethyl iodide and propyl iodide react with Na in the presence of ether, they form :
A)
two alkanes
done
clear
B)
four alkanes
done
clear
C)
three alkanes
done
clear
D)
one alkane
done
clear
View Answer play_arrow
question_answer 67) Froth floatation process is used in the metallurgy of:
A)
oxide ores
done
clear
B)
chloride ores
done
clear
C)
amalgams
done
clear
D)
sulphide ores
done
clear
View Answer play_arrow
question_answer 68) When chlorine reacts with ethyl alcohol, it forms:
A)
chloroform
done
clear
B)
chloral
done
clear
C)
ethyl chloride
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 69) The solubility of \[AgCl\] at \[{{20}^{o}}C\] is\[1.435\times {{10}^{-5}}g/L\]. The solubility product of \[AgCl\] is:
A)
\[108\times {{10}^{-3}}\]
done
clear
B)
\[1.0\times {{10}^{-14}}\]
done
clear
C)
\[2\times {{10}^{-16}}\]
done
clear
D)
\[1.035\times {{10}^{-5}}\]
done
clear
View Answer play_arrow
question_answer 70) Which of the following statements is correct for covalent bond?
A)
Its direction is polar
done
clear
B)
Electrons are shared between two atoms
done
clear
C)
Valency electrons are attracted in this bond
done
clear
D)
Its direction is non-polar.
done
clear
View Answer play_arrow
question_answer 71) Which of the following statement is correct?
A)
Phenol is more acidic than ethyl alcohol
done
clear
B)
Phenol is less acidic than ethyl alcohol
done
clear
C)
Phenol is more acidic than carboxylic acid
done
clear
D)
Phenol is more acidic than carbonic acid
done
clear
View Answer play_arrow
question_answer 72) Palmitic acid is:
A)
\[{{C}_{16}}{{H}_{31}}COOH\]
done
clear
B)
\[{{C}_{17}}{{H}_{35}}COOH\]
done
clear
C)
\[{{C}_{15}}{{H}_{31}}COOH\]
done
clear
D)
\[{{C}_{17}}{{H}_{31}}COOH\]
done
clear
View Answer play_arrow
question_answer 73) The values of heat of formation of \[S{{O}_{2}}\] and \[S{{O}_{3}}\] are 298.2 kJ and 98.2kJ. The heat of this reaction will be \[S{{O}_{2}}+1/2{{O}_{2}}\xrightarrow{{}}S{{O}_{3}}\]:
A)
- 396.2 kJ
done
clear
B)
-200 kJ
done
clear
C)
-356.2 kJ
done
clear
D)
+200kJ
done
clear
View Answer play_arrow
question_answer 74) If 0.15 g of a solute dissolved in 15 got solvent is boiled at a temperature higher by \[{{0.216}^{o}}C\], than that of the pure solvent, the molecular weight of the substance, (molal elevation constant for the solvent is \[{{2.16}^{o}}C\]):
A)
100
done
clear
B)
10.1
done
clear
C)
10
done
clear
D)
1.01
done
clear
View Answer play_arrow
question_answer 75) Which of the following is the IUPAC name of the given compound? \[C{{H}_{3}}-CH=\underset{\begin{smallmatrix} | \\ C{{H}_{2}}-C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
A)
3-methyl-3-pentene
done
clear
B)
2-ethyl-2-butene
done
clear
C)
- 3-methyl-2-pentene
done
clear
D)
3-ethyl-2-butene
done
clear
View Answer play_arrow
question_answer 76) Which of the following is not Lewis acid?
A)
\[FeC{{l}_{3}}\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[B{{F}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) What is correct sequence of bond order?
A)
\[{{O}_{2}}>{{O}_{2}}^{-}>{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}^{+}>{{O}_{2}}>{{O}_{2}}^{-}\]
done
clear
C)
\[{{O}_{2}}>{{O}_{2}}^{-}>{{O}_{2}}^{+}\]
done
clear
D)
\[{{O}_{2}}^{-}>{{O}_{2}}^{+}>{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 78) The metal present in Vitamin \[{{B}_{12}}\] is :
A)
cobalt
done
clear
B)
magnesium
done
clear
C)
manganese
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 79) Gibbs free energy for reaction at equilibrium is:
A)
\[+ve\]
done
clear
B)
\[-ve\]
done
clear
C)
zero
done
clear
D)
either [a] or [b]
done
clear
View Answer play_arrow
question_answer 80) \[{{D}_{2}}O\] (heavy water) is used in:
A)
chemical industry
done
clear
B)
insecticides preparation
done
clear
C)
nuclear reactor
done
clear
D)
pharmaceutical preparations
done
clear
View Answer play_arrow
question_answer 81) The rate constant for a first order reaction is\[k=7\times {{10}^{-4}}{{s}^{-1}}\]. The time taken for the reactant to be reduced to 1/4 of the initial concentration is:
A)
1980s
done
clear
B)
25905s
done
clear
C)
9905 s
done
clear
D)
4455 s
done
clear
View Answer play_arrow
question_answer 82) Bauxite is concentrated by :
A)
chemical method
done
clear
B)
roasting
done
clear
C)
magnetic separation
done
clear
D)
froth floatation process
done
clear
View Answer play_arrow
question_answer 83) Which is wrongly matched?
A)
Duralumin-\[Al+Cu+Mg+Mn\]
done
clear
B)
Ainico-\[Fe+Al+Ni+CO\]
done
clear
C)
German silver -\[Cu+Zn+Ni\]
done
clear
D)
Monel metal-\[Cu+Zn+Sn\]
done
clear
View Answer play_arrow
question_answer 84) Which of the following is coloured?
A)
\[T{{i}^{4+}}\]
done
clear
B)
\[{{V}^{5+}}\]
done
clear
C)
\[C{{u}^{+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 85) The size of particles, in suspension true solution and colloidal solution, varies in the order:
A)
suspension > colloidal > true solution
done
clear
B)
suspension > true solution > colloidal
done
clear
C)
true solution > suspension > colloidal
done
clear
D)
true solution > colloidal > suspension
done
clear
View Answer play_arrow
question_answer 86) Formula of green vitriol is :
A)
\[FeS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
B)
\[MgS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
C)
\[ZnS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
D)
\[CuS{{O}_{4}}\,.\,5{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 87) Ethyl alcohol gives ethyl chloride with the help of:
A)
\[SOC{{l}_{2}}\]
done
clear
B)
\[C{{l}_{2}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[KCl\]
done
clear
View Answer play_arrow
question_answer 88) The solubility product of \[A{{g}_{2}}Cr{{O}_{4}}\] is\[32\times {{10}^{-12}}\]. What is the concentration of \[{{[Cr{{O}_{4}}]}^{2-}}\] ions in that solution?
A)
\[8\times {{10}^{-8}}mol\,{{L}^{-1}}\]
done
clear
B)
\[16\times {{10}^{-4}}mol\,{{L}^{-1}}\]
done
clear
C)
\[2\times {{10}^{-4}}mol\,{{L}^{-1}}\]
done
clear
D)
\[8\times {{10}^{-4}}mol\,{{L}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 89) The change in optical rotation (with time) of freshly prepared solution of sugar is known as:
A)
inversion
done
clear
B)
anomers
done
clear
C)
mutarotation
done
clear
D)
epimers
done
clear
View Answer play_arrow
question_answer 90) Which of the following statement is correct?
A)
All halogens form oxy acid
done
clear
B)
Only chlorine and bromine form oxy acid
done
clear
C)
All halogens except fluorine form oxy acid
done
clear
D)
Only iodine form oxy acid
done
clear
View Answer play_arrow
question_answer 91) Equal volumes of 0.1 M \[HCl\] and \[0.1\,M\,NaOH\] are mixed. The concentration of the mixture will be:
A)
2M
done
clear
B)
0.05 M
done
clear
C)
0.02 M
done
clear
D)
0.1 M
done
clear
View Answer play_arrow
question_answer 92) Phenol can be converted into salicylic acid by:
A)
Cannizaros reaction
done
clear
B)
Perkins reaction
done
clear
C)
Tischenko reaction
done
clear
D)
Reimer-Tiemann reaction
done
clear
View Answer play_arrow
question_answer 93) The amount of electricity required to deposit 0.9 g of aluminium. When the electrode reaction is \[A{{l}^{3+}}+3{{e}^{-}}\to Al\]:
A)
\[9.65\times {{10}^{4}}C\]
done
clear
B)
\[4.34\times {{10}^{5}}C\]
done
clear
C)
\[9.65\times {{10}^{3}}C\]
done
clear
D)
\[1.93\times {{10}^{4}}C\]
done
clear
View Answer play_arrow
question_answer 94) Volume of gas at NTP is \[1.12\times {{10}^{-7}}cc\]. The number of molecules in the gas is :
A)
\[3.01\times {{10}^{12}}\]
done
clear
B)
\[30.1\times {{10}^{23}}\]
done
clear
C)
\[3.01\times {{10}^{24}}\]
done
clear
D)
\[3.01\times {{10}^{20}}\]
done
clear
View Answer play_arrow
question_answer 95) Calculate the standard heat of formation of carbon disulphide in liquid state. Given that the standard heat of combustion of carbon (s) sulphur (s) and carbon disulphide \[(l)\] are 393.3, - 29a72 and -1108.76 \[kJ\,mo{{l}^{-1}}\]respectively is:
A)
+ 128.02 \[kJ\,mo{{l}^{-1}}\]
done
clear
B)
-12802 \[kJ\,mo{{l}^{-1}}\]
done
clear
C)
+ 12.802 \[kJ\,mo{{l}^{-1}}\]
done
clear
D)
-128.02 \[kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) pH value of 0.01 M \[NaOH\] solution is:
A)
20
done
clear
B)
14
done
clear
C)
12
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 97) Which of the following elements has the maximum electron affinity?
A)
F
done
clear
B)
\[Cl\]
done
clear
C)
Br
done
clear
D)
I
done
clear
View Answer play_arrow
question_answer 98) The boiling points of the following hydrides follows the order of:
A)
\[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
B)
\[Sb{{H}_{3}}>N{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}\]
done
clear
C)
\[Sb{{H}_{3}}>As{{H}_{3}}>N{{H}_{3}}>P{{H}_{3}}\]
done
clear
D)
\[N{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 99) If \[HN{{O}_{3}}\] changes into \[{{N}_{2}}O\], the change in oxidation number is:
A)
zero
done
clear
B)
+4
done
clear
C)
+2
done
clear
D)
+ 6
done
clear
View Answer play_arrow
question_answer 100) The mass of an objects changes from 0.002 g to 0.00025 g in a time period of 1500 yr. The half life of the object is :
A)
1000 yr
done
clear
B)
700 yr
done
clear
C)
500 yr
done
clear
D)
250 yr
done
clear
View Answer play_arrow
question_answer 101) Retroviruses have :
A)
only RNA as genetic material
done
clear
B)
only DNA as genetic material
done
clear
C)
DNA and RNA as genetic material
done
clear
D)
genes on nucleoprotein material
done
clear
View Answer play_arrow
question_answer 102) Reindeer moss is a :
A)
pteridophyta
done
clear
B)
bryophyta
done
clear
C)
lichen
done
clear
D)
gymnosperm
done
clear
View Answer play_arrow
question_answer 103) Azolla is used as biofertilizers because it:
A)
has Rhizobium
done
clear
B)
is fast growing and can form humus
done
clear
C)
has cyanobacteria
done
clear
D)
has mycorrhiza
done
clear
View Answer play_arrow
question_answer 104) Lateral conjugation in Spirogyra occurs between individual which :
A)
are heterothallic
done
clear
B)
are homothallic
done
clear
C)
have different characters
done
clear
D)
are floating
done
clear
View Answer play_arrow
question_answer 105) Downs syndrome is characterised by:
A)
21st trisomy
done
clear
B)
two X and one Y chromosome
done
clear
C)
19 trisomy
done
clear
D)
only one X chromosome
done
clear
View Answer play_arrow
question_answer 106) Compositae is also called :
A)
Asteraceae
done
clear
B)
Myrtaceae
done
clear
C)
Fabaceae
done
clear
D)
Zingiberaceae
done
clear
View Answer play_arrow
question_answer 107) In plants translocation occurs in the form of:
A)
starch
done
clear
B)
sucrose
done
clear
C)
glucose
done
clear
D)
fructose
done
clear
View Answer play_arrow
question_answer 108) Which of the following is a component of electron transport chain in mitochondria?
A)
Phytochrome
done
clear
B)
Cytochrome oxidase
done
clear
C)
Plastocyanin
done
clear
D)
Carotenoids
done
clear
View Answer play_arrow
question_answer 109) The function of peroxisomes is:
A)
\[{{H}_{2}}{{O}_{2}}\] destruction
done
clear
B)
conversion of fat to carbohydrates
done
clear
C)
detoxification of heavy metals
done
clear
D)
oxidative phosphorylation
done
clear
View Answer play_arrow
question_answer 110) Cyanide resistant respiration is characteristic of:
A)
viruses
done
clear
B)
bacteria
done
clear
C)
plants
done
clear
D)
animals
done
clear
View Answer play_arrow
question_answer 111) Gibberellin was first isolated from :
A)
oat coleoptile
done
clear
B)
Actinomycete
done
clear
C)
fungus
done
clear
D)
soybean seedlings
done
clear
View Answer play_arrow
question_answer 112) Seedless water melons are obtained by :
A)
triploidy
done
clear
B)
gibberellin application
done
clear
C)
haploidy
done
clear
D)
vegetative propagation
done
clear
View Answer play_arrow
question_answer 113) Ethylene is involved in :
A)
aerobic respiration
done
clear
B)
anaerobic respiration
done
clear
C)
climacteric respiration
done
clear
D)
fermentation
done
clear
View Answer play_arrow
question_answer 114) The function of glyoxysome is :
A)
protein metabolism
done
clear
B)
carbohydrate metabolism
done
clear
C)
fat metabolism
done
clear
D)
protein synthesis
done
clear
View Answer play_arrow
question_answer 115) One gene-one enzyme theory was proposed by:
A)
Temin and Baltimore
done
clear
B)
Watson and Crick
done
clear
C)
Robert and Koch
done
clear
D)
Beadle and Tatum
done
clear
View Answer play_arrow
question_answer 116) Procambium forms :
A)
cork cambium
done
clear
B)
vascular tissue
done
clear
C)
vascular cambium
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow
question_answer 117) Cells having secretory function have abundant:
A)
lysosomes
done
clear
B)
endoplasmic reticulum
done
clear
C)
dictyosomes
done
clear
D)
osteosomes
done
clear
View Answer play_arrow
question_answer 118) Formation of RNA from DNA is known as :
A)
transcription
done
clear
B)
translation
done
clear
C)
replication
done
clear
D)
recombination
done
clear
View Answer play_arrow
question_answer 119) Which of the following cannot be isolated from plants?
A)
Riboflavin
done
clear
B)
Niacin
done
clear
C)
Vitamin \[{{B}_{12}}\]
done
clear
D)
Vitamin C
done
clear
View Answer play_arrow
question_answer 120) Stratification is seen in :
A)
Tundra
done
clear
B)
Tropical forest
done
clear
C)
Temperate forest
done
clear
D)
Desert
done
clear
View Answer play_arrow
question_answer 121) Relationship between different organisms can be best described by :
A)
blood web
done
clear
B)
pyramid of energy
done
clear
C)
pyramid of mass
done
clear
D)
Eltonian pyramid
done
clear
View Answer play_arrow
question_answer 122) Biogeochemical cycle of which element has atmospheric phase:
A)
carbon
done
clear
B)
sodium
done
clear
C)
phosphorus
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 123) Sieve tubes differ from sieve cells because they :
A)
have pores mainly on its walls
done
clear
B)
are shorter
done
clear
C)
lack nuclei
done
clear
D)
are dead
done
clear
View Answer play_arrow
question_answer 124) Cytokinins are formed in :
A)
roots
done
clear
B)
leaves
done
clear
C)
fruits
done
clear
D)
stems
done
clear
View Answer play_arrow
question_answer 125) Geotropic response is perceived by :
A)
root tip
done
clear
B)
root hairs
done
clear
C)
elongating cells
done
clear
D)
mature root cells
done
clear
View Answer play_arrow
question_answer 126) Ultimate source of variation is :
A)
mutation
done
clear
B)
mitosis
done
clear
C)
meiosis
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 127) Eggs of egg laying mammals are :
A)
macrolecithal
done
clear
B)
alecithal
done
clear
C)
mesolecithal
done
clear
D)
telolecithal
done
clear
View Answer play_arrow
question_answer 128) Mendelian recombinations are due to :
A)
linkage
done
clear
B)
independent assortment of genes
done
clear
C)
mutation
done
clear
D)
dominant characters
done
clear
View Answer play_arrow
question_answer 129) Which of the following is not a function of liver :
A)
Production of bile
done
clear
B)
Production of insulin
done
clear
C)
Glycogen storage
done
clear
D)
Detoxification
done
clear
View Answer play_arrow
question_answer 130) Ureotelic organisms:
A)
do not have urease
done
clear
B)
do not excrete urea
done
clear
C)
cannot form uric acid
done
clear
D)
live in water only
done
clear
View Answer play_arrow
question_answer 131) Rajaji national park is in:
A)
Karnataka
done
clear
B)
Rajasthan
done
clear
C)
UttarPradesh
done
clear
D)
Assam
done
clear
View Answer play_arrow
question_answer 132) Automobile exhaust causes respiratory problems because of the presence of:
A)
CO
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
chlorine
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 133) A plant which is cultivated by tissue culture technique is :
A)
Citrus
done
clear
B)
Apple
done
clear
C)
Pear
done
clear
D)
Guava
done
clear
View Answer play_arrow
question_answer 134) Cytokinin synthesis is maximum in :
A)
leaves
done
clear
B)
roots
done
clear
C)
fruits
done
clear
D)
shoot tip
done
clear
View Answer play_arrow
question_answer 135) Apnoea is:
A)
absence of breathing
done
clear
B)
decreased ventilation
done
clear
C)
increased ventilation
done
clear
D)
laboured breathing
done
clear
View Answer play_arrow
question_answer 136) Field capacity is associated with :
A)
capillary, hygroscopic, water vapour combined
done
clear
B)
capillary only
done
clear
C)
gravitational only
done
clear
D)
gravitational as well as
done
clear
View Answer play_arrow
question_answer 137) Food is translocated in plants through :
A)
xylem
done
clear
B)
phloem
done
clear
C)
cambium
done
clear
D)
cork
done
clear
View Answer play_arrow
question_answer 138) Which of the following organism is pseudocoelomate?
A)
Hookworm
done
clear
B)
Liver fluke
done
clear
C)
Jellyfish
done
clear
D)
Leech
done
clear
View Answer play_arrow
question_answer 139) Veliger larva is found in the phylum :
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Arthropoda
done
clear
D)
Cnidaria
done
clear
View Answer play_arrow
question_answer 140) Pick out the odd one :
A)
Aniielida
done
clear
B)
Reptilia
done
clear
C)
Insecta
done
clear
D)
Taenia
done
clear
View Answer play_arrow
question_answer 141) Which of the following statement about bee colony is wrong?
A)
Domesticated species are A. indica and A. mellifera
done
clear
B)
Sex differentiation is due to haplodiploidy
done
clear
C)
They are female dominant societies
done
clear
D)
Fertilized eggs develop into sterile females only
done
clear
View Answer play_arrow
question_answer 142) Calmodulin is a :
A)
chlorophyll binding protein
done
clear
B)
cadmium binding protein
done
clear
C)
calcium binding protein
done
clear
D)
carotene binding protein
done
clear
View Answer play_arrow
question_answer 143) Potato is native to :
A)
South America
done
clear
B)
North America
done
clear
C)
South East
done
clear
D)
Australia
done
clear
View Answer play_arrow
question_answer 144) Bacterial mutants requiring special nutrients are known as :
A)
prototrophs
done
clear
B)
autotrophs
done
clear
C)
heterotrophs
done
clear
D)
auxotrophs
done
clear
View Answer play_arrow
question_answer 145) Which of the following is not secreted by anterior pituitary?
A)
Prolactin
done
clear
B)
FSH
done
clear
C)
Growth hormone
done
clear
D)
ADH
done
clear
View Answer play_arrow
question_answer 146) Book lungs are respiratory organs of:
A)
Mollusca
done
clear
B)
Mammals
done
clear
C)
Arachnida
done
clear
D)
Annelida
done
clear
View Answer play_arrow
question_answer 147) Cranial nerves numbering IV, V and VI are respectively:
A)
trochlear, trigeminal, abducens
done
clear
B)
trochlear, trigeminal, facial
done
clear
C)
auditory, facial, trochlear
done
clear
D)
auditory, trochlear, facial
done
clear
View Answer play_arrow
question_answer 148) In ruminants cellulose digestion takes place by:
A)
bacteria
done
clear
B)
protozoans
done
clear
C)
virus
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 149) Which of the following organism is useful in degrading organic pollutants :
A)
Nitrosomonas
done
clear
B)
Chlamydomonas
done
clear
C)
Actinomycetes
done
clear
D)
Pseudomonas
done
clear
View Answer play_arrow
question_answer 150) Organisms found in extreme temperature are:
A)
cyanobacteria
done
clear
B)
archaebacteria
done
clear
C)
fungi
done
clear
D)
eubacteria
done
clear
View Answer play_arrow
question_answer 151) Which of the following are the characteristics of deuterosome ?
A)
Spiral cleavage, blastopore becoming anus
done
clear
B)
Spiral cleavage, blastopore becoming mouth
done
clear
C)
Radial cleavage, blastopore becoming anus
done
clear
D)
Radial cleavage, blastopore becoming mouth
done
clear
View Answer play_arrow
question_answer 152) Emulsification is the function of :
A)
bile
done
clear
B)
lipases
done
clear
C)
esterases
done
clear
D)
proteases
done
clear
View Answer play_arrow
question_answer 153) Which of the following is a monocarpic plant?
A)
Bambusa
done
clear
B)
Sesamum
done
clear
C)
Lauandual
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 154) Cotton is:
A)
epidermal tissue system
done
clear
B)
vascular tissue system
done
clear
C)
meristematic tissue system
done
clear
D)
ground tissue-system
done
clear
View Answer play_arrow
question_answer 155) Marine protozoans lack contractile vacuole because:
A)
osmoregulation is done by cell membrane
done
clear
B)
they are hypoosmotic to their environment
done
clear
C)
they are isotonic with their environment
done
clear
D)
their small body cannot accommodate it
done
clear
View Answer play_arrow
question_answer 156) The maintenance of internal favourable conditions, by a self regulated mechanism causing changes in environment is:
A)
homeostasis
done
clear
B)
steady state
done
clear
C)
cyclosis
done
clear
D)
ecosystem
done
clear
View Answer play_arrow
question_answer 157) Ontogeny recapitulates phylogeny. This is :
A)
Pauling law
done
clear
B)
Hardy Weinberg law
done
clear
C)
Biogenetic law
done
clear
D)
Thomas law
done
clear
View Answer play_arrow
question_answer 158) DNA strands :
A)
have quartenary structure like protein
done
clear
B)
are bonded by disulphide bonds
done
clear
C)
have some polarity
done
clear
D)
are antiparallel
done
clear
View Answer play_arrow
question_answer 159) Endoplasmic reticulum is studied with :
A)
ribosomes
done
clear
B)
lysosomes
done
clear
C)
glyoxisomes
done
clear
D)
microsomes
done
clear
View Answer play_arrow
question_answer 160) Heavy metals are detoxified in plants by :
A)
abscisic acid
done
clear
B)
alleopathins
done
clear
C)
phytochelatins
done
clear
D)
phytoalexins
done
clear
View Answer play_arrow
question_answer 161) Which of the following is not derived from ectoderm?
A)
Inner ear
done
clear
B)
Middle ear
done
clear
C)
Optic nerve
done
clear
D)
Skin
done
clear
View Answer play_arrow
question_answer 162) Predation and parasitism are which type of interactions :
A)
(+,+)
done
clear
B)
(+,0)
done
clear
C)
(-,-)
done
clear
D)
(+,-)
done
clear
View Answer play_arrow
question_answer 163) Unwinding of DNA is done by :
A)
primase
done
clear
B)
exonuclease
done
clear
C)
helicase
done
clear
D)
ligase
done
clear
View Answer play_arrow
question_answer 164) Plants require sulphur for :
A)
DNA replication
done
clear
B)
protein synthesis
done
clear
C)
synthesis of glucose
done
clear
D)
formation of ATP
done
clear
View Answer play_arrow
question_answer 165) Fungi differ from other kingdom in being :
A)
unicellular
done
clear
B)
unicellular, consumers
done
clear
C)
multicellular, decomposers
done
clear
D)
multicellular, consumer
done
clear
View Answer play_arrow
question_answer 166) Confusion technique involves the use of:
A)
juvenile hormone
done
clear
B)
pheromones
done
clear
C)
ecdysone
done
clear
D)
hormones
done
clear
View Answer play_arrow
question_answer 167) A nonsense mutation results into :
A)
stoppage of transcription
done
clear
B)
change in protein structure
done
clear
C)
stoppage of protein synthesis
done
clear
D)
termination of polypeptide chain
done
clear
View Answer play_arrow
question_answer 168) Bone marrow does not occur in:
A)
amphibians
done
clear
B)
birds
done
clear
C)
reptilia
done
clear
D)
apes
done
clear
View Answer play_arrow
question_answer 169) Menstrual How occurs due to lack of
A)
progesterone
done
clear
B)
FSH
done
clear
C)
oxytocin
done
clear
D)
vasopressin
done
clear
View Answer play_arrow
question_answer 170) In vegetative propagation of the tubers which of the following remain constant through generations ?
A)
Morphology, vigour and disease resistance
done
clear
B)
Morphology only
done
clear
C)
Vigour only
done
clear
D)
Vigour and morphology only
done
clear
View Answer play_arrow
question_answer 171) Which one caused mutation in animals?
A)
Change in habit
done
clear
B)
Change in Golgi body
done
clear
C)
Change in gene structure
done
clear
D)
Change in peroxysome
done
clear
View Answer play_arrow
question_answer 172) Electron microscope was discovered by :
A)
M. Knoll and E Ruska
done
clear
B)
Robertson
done
clear
C)
Robert Hooke
done
clear
D)
Lehmann
done
clear
View Answer play_arrow
question_answer 173) The stem of Equisetum is rough due to :
A)
spines
done
clear
B)
silica
done
clear
C)
sclerids
done
clear
D)
fibres
done
clear
View Answer play_arrow
question_answer 174) Sinus venosus is present in :
A)
birds only
done
clear
B)
birds and mammals
done
clear
C)
reptiles and birds
done
clear
D)
fishes, amphibians and reptiles
done
clear
View Answer play_arrow
question_answer 175) Which of the following is not a characteristic of Echinoderm?
A)
Endodermal skeletal system
done
clear
B)
Water vascular system
done
clear
C)
Bilateral symmetry
done
clear
D)
Free floating larval forms
done
clear
View Answer play_arrow
question_answer 176) Funaria and Marchantia differ from each other because Funaria posses :
A)
ventral canal cell
done
clear
B)
foot
done
clear
C)
calyptra
done
clear
D)
protonema
done
clear
View Answer play_arrow
question_answer 177) Ducts of Bellini are present in :
A)
medulla oblongata
done
clear
B)
liver
done
clear
C)
kidney
done
clear
D)
intestine
done
clear
View Answer play_arrow
question_answer 178) Cow dung is appropriately used as :
A)
fuel
done
clear
B)
building material
done
clear
C)
medicine
done
clear
D)
manure
done
clear
View Answer play_arrow
question_answer 179) Protein packaging is done by :
A)
Golgi apparatus
done
clear
B)
ribosomes
done
clear
C)
end oplasmic reticulum
done
clear
D)
nucleolus
done
clear
View Answer play_arrow
question_answer 180) The shape of eye lens is changed by :
A)
pupil
done
clear
B)
iris
done
clear
C)
optic nerve
done
clear
D)
ciliary muscles
done
clear
View Answer play_arrow
question_answer 181) Eutrophication is seen in :
A)
saline soil
done
clear
B)
agricultural land near thermal plant
done
clear
C)
lake
done
clear
D)
mountain
done
clear
View Answer play_arrow
question_answer 182) Which of the following are viral and mosquito borne disease?
A)
Filariasis and typhus
done
clear
B)
Kala azar and diphtheria
done
clear
C)
Malaria and chagas disease
done
clear
D)
Yellow fever and dengue
done
clear
View Answer play_arrow
question_answer 183) Chemofusion and dectrofusion are characteristic of:
A)
mutations
done
clear
B)
cloning
done
clear
C)
protoplast fusion
done
clear
D)
eugenics
done
clear
View Answer play_arrow
question_answer 184) 1 : 2 : 1 phenotypic and genotypic ratio is found in :
A)
complementary genes
done
clear
B)
blending inheritance
done
clear
C)
multiple alleles
done
clear
D)
pseudo alleles
done
clear
View Answer play_arrow
question_answer 185) Tasmanian wolf is a marsupial while wolf is a placental mammal. This shows:
A)
convergent evolution
done
clear
B)
divergent evolution
done
clear
C)
parallism
done
clear
D)
inheritance of acquired characters
done
clear
View Answer play_arrow
question_answer 186) Which of the following is a basic amino acid?
A)
Leucine
done
clear
B)
Lysine
done
clear
C)
Methionine
done
clear
D)
Aspartic acid
done
clear
View Answer play_arrow
question_answer 187) Which of the following is an endangered animal?
A)
Hanuman monkey
done
clear
B)
Langur
done
clear
C)
Antelope
done
clear
D)
Lion tailed macaque
done
clear
View Answer play_arrow
question_answer 188) Which of the following statements about blood transfusion is correct. Blood group B can give blood to?
A)
Blood groups B, AB and receive from group B
done
clear
B)
Blood group B and receive from group AB
done
clear
C)
Blood group O and receive from group B
done
clear
D)
Blood group B, AB and receive from group AB
done
clear
View Answer play_arrow
question_answer 189) DNA synthesis occurs in :
A)
prometaphase
done
clear
B)
prophase
done
clear
C)
interphase
done
clear
D)
telophase
done
clear
View Answer play_arrow
question_answer 190) A cell is heterozygous at three gene loci. How many different type of gametes can it form?
A)
2
done
clear
B)
3
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 191) Heterozygosity is most favoured in plants following:
A)
cleistogamy
done
clear
B)
autogamy
done
clear
C)
xenogamy
done
clear
D)
geitonogamy
done
clear
View Answer play_arrow
question_answer 192) In a certain plant red colour flower (R) is dominant over white colour flower (r). When heterozygous Rr plant is crossed 64 offsprings are obtained. The number of white offsprings are:
A)
64
done
clear
B)
0
done
clear
C)
16
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 193) Cancer of prostate gland is caused due to the exposure to:
A)
lead oxide
done
clear
B)
cadmium oxide
done
clear
C)
potassium oxide
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 194) The presence of recessive part in a large population is found to be 16%. The frequency of dominant allele in that population is :
A)
0.84
done
clear
B)
0.32
done
clear
C)
0.56
done
clear
D)
0.92
done
clear
View Answer play_arrow
question_answer 195) When a person is exposed to cold surrounding, which of the following does not occur ?
A)
Secretion of adrenal medulla and thyroid increase
done
clear
B)
Shivering occurs
done
clear
C)
Vasoconstricdon in skin vessels supplying blood to hairs
done
clear
D)
Heart beat and volume of blood per stoke increase
done
clear
View Answer play_arrow
question_answer 196) Which of the following is a pollutant from automobiles?
A)
CO
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 197) From which of the following plants a medicine for respiratory disorders is obtained?
A)
Ephedra
done
clear
B)
Eucalyptus
done
clear
C)
Cannabis
done
clear
D)
Saccharum
done
clear
View Answer play_arrow
question_answer 198) The habitat of a species along with its function is :
A)
home range
done
clear
B)
tersitory
done
clear
C)
boundary
done
clear
D)
niche
done
clear
View Answer play_arrow
question_answer 199) Which of the following statement about Lamarck is true ?
A)
He was a French botanist who late become Zoologists
done
clear
B)
He was an English naturalist who gave theory of evolution
done
clear
C)
He was a polish scientist and gave law of inheritance
done
clear
D)
He was a French scientist who gave inheritance by acquired characters
done
clear
View Answer play_arrow
question_answer 200) Two places in India show maximum biological diversity. One of them is Western ghat another is:
A)
Eastern ghat
done
clear
B)
Coastal region
done
clear
C)
North-East India
done
clear
D)
Foot hills of Garhwal region
done
clear
View Answer play_arrow