-
question_answer1) A pair of physical quantities having same dimensional formula is:
A)
force and torque
done
clear
B)
Work and energy
done
clear
C)
force and impulse
done
clear
D)
linear momentum and angular momentum
done
clear
View Answer play_arrow
-
question_answer2) An engine exerts a force \[\vec{F}=(20\hat{i}-3\hat{j}+5\hat{k})N\]and moves with velocity \[\vec{v}=(6\hat{j}+20\hat{j}-3\hat{k})\,m/s\]. The power of the engine (in watt) is:
A)
45
done
clear
B)
75
done
clear
C)
20
done
clear
D)
10
done
clear
View Answer play_arrow
-
question_answer3) Two particles are projected with same initial velocities at an angle \[{{30}^{\text{o}}}\] and \[{{60}^{\text{o}}}\] with the horizontal. Then:
A)
their heights will be equal
done
clear
B)
their ranges will be equal
done
clear
C)
their time of flights will be equal
done
clear
D)
their ranges will be different
done
clear
View Answer play_arrow
-
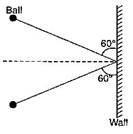
question_answer4) A ball of mass 3 kg moving with a speed of 100 m/s, strikes a wall at an angle \[{{60}^{\text{o}}}\] (as shown in figure). The ball rebounds at the same speed and remains in contact with the ball for 0.2 s, the force exerted by the ball on the wall is:
A)
\[1500\sqrt{3}\,N\]
done
clear
B)
1500 N
done
clear
C)
\[300\,\sqrt{3}\,N\]
done
clear
D)
300 N
done
clear
View Answer play_arrow
-
question_answer5)
Two masses \[{{M}_{1}}=5\,\,kg,\,\,{{M}_{2}}=10\,kg\] are connected at the ends of an inextensible string passing over a frictionless pulley as shown. When masses are released, then acceleration of masses will be: 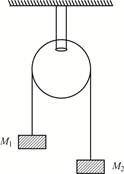
A)
g
done
clear
B)
\[\frac{g}{2}\]
done
clear
C)
\[\frac{g}{3}\]
done
clear
D)
\[\frac{g}{4}\]
done
clear
View Answer play_arrow
-
question_answer6) A particle moves along a straight line such that its displacement at any time t is given by \[s=3{{t}^{3}}+7{{t}^{2}}+14t+5\]. The acceleration of the particle at t = 1 s is:
A)
\[18\,\,m/{{s}^{2}}\]
done
clear
B)
\[32\,\,m/{{s}^{2}}\]
done
clear
C)
\[29\,m/{{s}^{2}}\]
done
clear
D)
\[24\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer7) A boat which has a speed of 5 km/h in still water crosses a river of width 1 km along the shortest possible path in 15 min. The velocity of the river water in km/h is:
A)
1
done
clear
B)
3
done
clear
C)
4
done
clear
D)
\[\sqrt{41}\]
done
clear
View Answer play_arrow
-
question_answer8) A particle of mass 1 kg is thrown vertically upwards with speed 100 m/s. After 5 s it explodes into two parts. One part of mass 400 g comes back with speed 25 m/s, what is the speed of other part just after explosion?
A)
100 m/s upward
done
clear
B)
600 m/s upward
done
clear
C)
100 m/s downward
done
clear
D)
300 m/s upward
done
clear
View Answer play_arrow
-
question_answer9)
A stone is attached to one end of a string and rotated in a vertical circle. If string breaks at the position of maximum tension, it will break at: 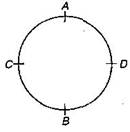
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
-
question_answer10) A solid sphere and a hollow sphere are thrown horizontally from a cliff with equal velocities, respectively. Then which sphere reaches first on earth?
A)
Solid sphere
done
clear
B)
Hollow sphere
done
clear
C)
Both sphere simultaneously
done
clear
D)
We cannot say because masses of spheres are not given
done
clear
View Answer play_arrow
-
question_answer11) Escape velocity from earth is 11.2 km/s. Another planet of same mass has radius 1/4 times that of earth. What is the escape velocity from another planet?
A)
11.2 km/s
done
clear
B)
44.8 km/s
done
clear
C)
22.4 km/s
done
clear
D)
5.6 km/s
done
clear
View Answer play_arrow
-
question_answer12)
ABC is a right angled triangular plate of uniform thickness. The sides are such that AB > BC as shown in figure. \[{{I}_{1}},\,{{I}_{2}},\,{{I}_{3}}\] are moments of inertia about AB, BC and AC respectively. Then which of the following relations is correct? 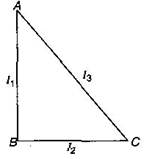
A)
\[{{I}_{1}}={{I}_{2}}={{I}_{3}}\]
done
clear
B)
\[{{I}_{2}}>{{I}_{1}}>{{I}_{3}}\]
done
clear
C)
\[{{I}_{3}}<{{I}_{2}}<{{I}_{1}}\]
done
clear
D)
\[{{I}_{3}}>{{I}_{1}}>{{I}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer13) A gas is formed of molecules each molecule possessing \[f\] degrees of freedom, then the value of \[\gamma =\frac{{{C}_{P}}}{{{C}_{V}}}\] is equal to:
A)
\[\frac{2}{f}\]
done
clear
B)
\[1+\frac{2}{f}\]
done
clear
C)
\[1+\frac{f}{2}\]
done
clear
D)
\[f+\frac{1}{2}\]
done
clear
View Answer play_arrow
-
question_answer14) A pendulum is displaced to an angle \[\theta \] from its equilibrium position; then it will pass through its mean position with a velocity v equal to:
A)
\[\sqrt{2gl}\]
done
clear
B)
\[\sqrt{2gl\sin \theta }\]
done
clear
C)
\[\sqrt{2gl\cos \theta }\]
done
clear
D)
\[\sqrt{2gl\,\,(1-\cos \theta )}\]
done
clear
View Answer play_arrow
-
question_answer15) An engine takes heat from a reservoir and converts its 1/6 part into work. By decreasing temperature of sink by \[{{62}^{\text{o}}}C,\] its efficiency becomes double. The temperatures of source and sink must be:
A)
\[{{90}^{\text{o}}}C,\,{{37}^{\text{o}}}C\]
done
clear
B)
\[{{99}^{\text{o}}}C,\,{{37}^{\text{o}}}C\]
done
clear
C)
\[{{372}^{\text{o}}}C,\,{{37}^{\text{o}}}C\]
done
clear
D)
\[{{206}^{\text{o}}}C,\,{{37}^{\text{o}}}C\]
done
clear
View Answer play_arrow
-
question_answer16) Two sources are at a finite distance apart. They emit sounds of wavelength \[\lambda \]. An observer situated between them on line joining approaches one source with speed \[u\]. Then the number of beats heard/s by observer will be:
A)
\[\frac{2u}{\lambda }\]
done
clear
B)
\[\frac{u}{\lambda }\]
done
clear
C)
\[\frac{u}{2\lambda }\]
done
clear
D)
\[\frac{\lambda }{u}\]
done
clear
View Answer play_arrow
-
question_answer17) Rainbows are formed by:
A)
reflection and diffraction
done
clear
B)
refraction and scattering
done
clear
C)
dispersion and total internal reflection
done
clear
D)
interference only
done
clear
View Answer play_arrow
-
question_answer18) A man is 6 feet tall. In order to see his entire image, he requires a plane mirror of minimum length equal to:
A)
6 ft
done
clear
B)
12 ft
done
clear
C)
2 ft
done
clear
D)
3 ft
done
clear
View Answer play_arrow
-
question_answer19) Two simple harmonic motions given by \[x=A\sin (\omega t+\delta )\] and \[y=A\,\sin \left( \omega t+\delta +\frac{\pi }{2} \right)\] act on a particle simultaneously; then the motion of particle will be:
A)
circular anti-clockwise
done
clear
B)
circular clockwise
done
clear
C)
elliptical anti-clockwise
done
clear
D)
elliptical clockwise
done
clear
View Answer play_arrow
-
question_answer20) A plano-convex lens is made of a material of refractive index \[\mu =1.5\]. The radius of curvature of curved surface of the lens is 20 cm. If its plane surface is silvered, the focal length of the silvered lens will be:
A)
10 cm
done
clear
B)
20 cm
done
clear
C)
40 cm
done
clear
D)
80 cm
done
clear
View Answer play_arrow
-
question_answer21) Two wires are held perpendicular to the plane of paper at 5 m apart. They carry currents of 2.5 A and 5 A in same direction. Then the magnetic field strength (b) at a point midway between the wires will be:
A)
\[\frac{{{\mu }_{0}}}{4\pi }T\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{2\pi }T\]
done
clear
C)
\[\frac{3{{\mu }_{0}}}{2\pi }T\]
done
clear
D)
\[\frac{3{{\mu }_{0}}}{4\pi }T\]
done
clear
View Answer play_arrow
-
question_answer22) A capacitor is charged by connecting a battery across its plates. It stores energy U. Now the battery is disconnected and another identical capacitor is connected across it, then the energy stored by both capacitors of the system will be:
A)
U
done
clear
B)
\[\frac{U}{2}\]
done
clear
C)
2U
done
clear
D)
\[\frac{3}{2}U\]
done
clear
View Answer play_arrow
-
question_answer23)
A bridge circuit is shown in figure the equivalent resistance between A and B will be: 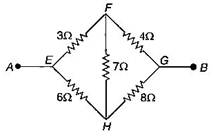
A)
\[21\,\Omega \]
done
clear
B)
\[7\Omega \]
done
clear
C)
\[\frac{252}{85}\Omega \]
done
clear
D)
\[\frac{14}{3}\Omega \]
done
clear
View Answer play_arrow
-
question_answer24) Two bulbs 25 W, 220 V and 100 W, 220 V are given. Which has higher resistance?
A)
25 W bulb
done
clear
B)
100 W bulb
done
clear
C)
Both bulbs will have equal resistance
done
clear
D)
Resistance of bulbs cannot be compared
done
clear
View Answer play_arrow
-
question_answer25) An electron moves with a velocity \[1\times {{10}^{3}}\,m/s\] in a magnetic field of induction 0.3 T at an angle \[{{30}^{o}}\]. If \[\frac{e}{m}\] of electron is \[1.76\times {{10}^{11}}\,C/kg\] the radius of the path is nearly:
A)
\[{{10}^{-8}}\,m\]
done
clear
B)
\[2\times {{10}^{-8}}\,m\]
done
clear
C)
\[{{10}^{-6}}\,m\]
done
clear
D)
\[{{10}^{-10}}\,m\]
done
clear
View Answer play_arrow
-
question_answer26) A charged wire is bent in the form of a semi-circular arc of radius a. If charge per unit length is \[\lambda \] coulomb/metre, the electric field at the centre O is:
A)
\[\frac{\lambda }{2\pi {{a}^{2}}{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{\lambda }{4{{\pi }^{2}}{{\varepsilon }_{0}}a}\]
done
clear
C)
\[\frac{\lambda }{2{{\pi }^{2}}{{\varepsilon }_{0}}a}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
-
question_answer27) Potentiometer measures the potential difference more accurately than a voltmeter because:
A)
it has a wire of high resistance
done
clear
B)
it has a wire of low resistance
done
clear
C)
it does not draw current from external circuit
done
clear
D)
it draws a heavy current from external circuit
done
clear
View Answer play_arrow
-
question_answer28) In electrolysis the mass deposited on an electrode is directly proportional to:
A)
current
done
clear
B)
square of current
done
clear
C)
concentration of solution
done
clear
D)
inverse of current
done
clear
View Answer play_arrow
-
question_answer29) The frequency of \[\gamma -rays,\] X-rays and ultraviolet rays are a, b and c respectively. Then:
A)
\[a>b>c\]
done
clear
B)
\[a<b<c\]
done
clear
C)
\[a=b=c\]
done
clear
D)
\[a>c>b\]
done
clear
View Answer play_arrow
-
question_answer30) A wire of resistance R is connected in series with an inductor of reactance \[\omega L\]. Then quality factor of RL circuit is:
A)
\[\frac{R}{\omega L}\]
done
clear
B)
\[\frac{\omega L}{R}\]
done
clear
C)
\[\frac{R}{\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}}\]
done
clear
D)
\[\frac{\omega L}{\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}}\]
done
clear
View Answer play_arrow
-
question_answer31) Einstein's work on photoelectric effect gives support to:
A)
\[E=m{{c}^{2}}\]
done
clear
B)
E = hv
done
clear
C)
\[hv=\frac{1}{2}m{{v}^{2}}\]
done
clear
D)
\[E=\frac{h}{\lambda }\]
done
clear
View Answer play_arrow
-
question_answer32) Nuclear fission can be explained by:
A)
proton-proton cycle
done
clear
B)
liquid drop model of nucleus
done
clear
C)
independent of nuclear particle model
done
clear
D)
nuclear shell model
done
clear
View Answer play_arrow
-
question_answer33) When electron jumps from n = 4 to n = 2 orbit, we get:
A)
second line of Lyman series
done
clear
B)
second line of Balmer series
done
clear
C)
second line of Paschen series
done
clear
D)
an absorption line of Balmer series
done
clear
View Answer play_arrow
-
question_answer34) Which of the following transitions gives photon of maximum energy?
A)
n = 1 to n = 2
done
clear
B)
n = 2 to n = 1
done
clear
C)
n = 2 to n = 6
done
clear
D)
n = 6 to n = 2
done
clear
View Answer play_arrow
-
question_answer35) The relationship between disintegration constant \[(\lambda )\] and half-life \[(T)\] will be:
A)
\[\lambda =\frac{{{\log }_{10}}2}{T}\]
done
clear
B)
\[\lambda =\frac{{{\log }_{e}}2}{T}\]
done
clear
C)
\[\lambda =\frac{T}{{{\log }_{e}}2}\]
done
clear
D)
\[\lambda =\frac{{{\log }_{2}}e}{T}\]
done
clear
View Answer play_arrow
-
question_answer36) The half-life of a radioactive material is 3 h. If the initial amount is 300 g, then after 18 h, it will remain:
A)
4.68 g
done
clear
B)
46.8 g
done
clear
C)
9.375 g
done
clear
D)
93.75 g
done
clear
View Answer play_arrow
-
question_answer37) A nuclear decay is expressed as \[_{6}{{C}^{11}}\to {{\,}_{5}}{{B}^{11}}+\,{{\beta }^{+}}+X\] Then the unknown particle X is:
A)
neutron
done
clear
B)
antineutrino
done
clear
C)
proton
done
clear
D)
neutrino
done
clear
View Answer play_arrow
-
question_answer38) If \[\alpha \] and \[\beta \] are current gains in common-base and common-emitter configurations of a transistor, then \[\beta \] is equal to:
A)
\[\frac{1}{\alpha }\]
done
clear
B)
\[\frac{\alpha }{1+\alpha }\]
done
clear
C)
\[\frac{\alpha }{1-\alpha }\]
done
clear
D)
\[\alpha -\frac{1}{\alpha }\]
done
clear
View Answer play_arrow
-
question_answer39)
The truth table given below: | Inputs | Output |
| A | B | Y |
| 0 | 0 | 0 |
| 1 | 0 | 0 |
| 0 | 1 | 0 |
| 1 | 1 | 1 |
represents:
A)
AND gate
done
clear
B)
NOR gate
done
clear
C)
OR gate
done
clear
D)
NAND gate
done
clear
View Answer play_arrow
-
question_answer40) The gases carbon-monoxide (CO) and nitrogen at the same temperature have kinetic energies \[{{E}_{1}}\] and \[{{E}_{2}}\] respectively. Then:
A)
\[{{E}_{1}}={{E}_{2}}\]
done
clear
B)
\[{{E}_{1}}>{{E}_{2}}\]
done
clear
C)
\[{{E}_{1}}<{{E}_{2}}\]
done
clear
D)
\[{{E}_{1}}\] and \[{{E}_{2}}\] cannot be compared
done
clear
View Answer play_arrow
-
question_answer41) A sonometer wire when vibrated in full length has frequency n. Now it is divided by the help of bridges into a number of segments of lengths \[{{l}_{1}},\,{{l}_{2}},\,{{l}_{3}}...\]. When vibrated these segments have frequencies \[{{n}_{1}},\,{{n}_{2}},\,{{n}_{3}},....\]then the correct relation is:
A)
\[n={{n}_{1}}+{{n}_{2}}+{{n}_{3}}+...\]
done
clear
B)
\[{{n}^{2}}=n_{1}^{2}+n_{2}^{2}+n_{3}^{2}+...\]
done
clear
C)
\[\frac{1}{n}=\frac{1}{{{n}_{1}}}+\frac{1}{{{n}_{2}}}+\frac{1}{{{n}_{3}}}+...\]
done
clear
D)
\[\frac{1}{\sqrt{n}}=\frac{1}{\sqrt{{{n}_{1}}}}+\frac{1}{\sqrt{{{n}_{2}}}}+\frac{1}{\sqrt{{{n}_{3}}}}+...\]
done
clear
View Answer play_arrow
-
question_answer42) In which of the following figures, junction diode is forward biased?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer43) The energy of a photon of light is 3 eV. Then the wavelength of photon must be:
A)
4125 nm
done
clear
B)
412.5 nm
done
clear
C)
41250 nm
done
clear
D)
4 nm
done
clear
View Answer play_arrow
-
question_answer44) A body has a weight 72 N. When it is taken to a height \[h=R\] (radius of earth), it would weigh:
A)
72 N
done
clear
B)
36 N
done
clear
C)
18 N
done
clear
D)
zero
done
clear
View Answer play_arrow
-
question_answer45) A cell has an emf 1.5 V. When connected across an external resistance of \[2\,\Omega \], the terminal potential difference falls to 1.0 V. The internal resistance of the cell is:
A)
\[2\,\Omega \]
done
clear
B)
\[1.5\,\Omega \]
done
clear
C)
\[1.0\,\Omega \]
done
clear
D)
\[0.5\,\Omega \]
done
clear
View Answer play_arrow
-
question_answer46) A charge q is placed at the corner of a cube of side \[a\]. The electric flux through the cube is:
A)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{q}{3{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{q}{6\,{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{q}{8\,{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
-
question_answer47) A man goes at the top of a smooth inclined plane. He releases a bag to fall freely and he himself slides on inclined plane to reach the bottom. If \[{{v}_{1}}\] and \[{{v}_{2}}\] are the velocities of the man and bag respectively, then:
A)
\[{{v}_{1}}>{{v}_{2}}\]
done
clear
B)
\[{{v}_{1}}<{{v}_{2}}\]
done
clear
C)
\[{{v}_{1}}={{v}_{2}}\]
done
clear
D)
\[{{v}_{1}}\] and \[{{v}_{2}}\] cannot be compared
done
clear
View Answer play_arrow
-
question_answer48) A transparent cube contains a small air bubble. Its apparent distance is 2 cm when seen through one face and 5 cm when seen through other face. If the refractive index of the material of the cube is 1.5, the real length of the edge of cube must be:
A)
7 cm
done
clear
B)
7.5 cm
done
clear
C)
10.5 cm
done
clear
D)
\[\frac{14}{3}\]cm
done
clear
View Answer play_arrow
-
question_answer49) Which one of the following processes depends on gravity?
A)
Conduction
done
clear
B)
Convection
done
clear
C)
Radiation
done
clear
D)
None
done
clear
View Answer play_arrow
-
question_answer50) Two strings A and B have lengths \[{{l}_{A}}\] and \[{{l}_{B}}\] and carry masses \[{{M}_{A}}\] and \[{{M}_{B}}\] at their lower ends, the upper ends being supported by rigid supports. If \[{{n}_{A}}\] and \[{{n}_{B}}\] are their frequencies of their vibrations and \[{{n}_{A}}=2{{n}_{B}},\] then:
A)
\[{{l}_{A}}=4{{l}_{B}}\], regardless of masses
done
clear
B)
\[{{l}_{B}}=4{{l}_{A}}\], regardless of masses
done
clear
C)
\[{{M}_{A}}=2{{M}_{B}},\,{{l}_{A}}=2{{l}_{B}}\]
done
clear
D)
\[{{M}_{B}}=2{{M}_{A}},\,{{l}_{B}}=2{{l}_{A}}\]
done
clear
View Answer play_arrow
-
question_answer51) The energy of photon is given as: \[\Delta e\]/atom = \[3.03\times {{10}^{-19}}\,J\,ato{{m}^{-1}}\] then, the wavelength \[(\lambda )\] of the photon is:
A)
6.56 nm
done
clear
B)
65.6 nm
done
clear
C)
656 nm
done
clear
D)
0,656 nm (Given, h(Planck's constant) \[=6.63\,\times {{10}^{-34}}\,J-s,\] c(velocity of light) \[=3.00\,\times {{10}^{8}}\,m{{s}^{-1}}\])
done
clear
View Answer play_arrow
-
question_answer52) Which one of the following is planar?
A)
\[Xe{{F}_{4}}\]
done
clear
B)
\[Xe{{O}_{4}}\]
done
clear
C)
\[Xe{{O}_{3}}F\] \[Xe{{O}_{3}}F\]
done
clear
D)
\[Xe{{O}_{3}}{{F}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer53) The conjugate acid of \[NH_{2}^{-}\] is:
A)
\[{{N}_{2}}{{H}_{4}}\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer54) The equivalent conductances of \[B{{a}^{2+}}\] and \[C{{l}^{-}}\] are 127 and 76 \[oh{{m}^{-1}}\,\,c{{m}^{-1}}\,e{{q}^{-1}}\] respectively at infinite dilution. The equivalent conductance of \[BaC{{l}_{2}}\] at infinite dilution will be:
A)
139.5
done
clear
B)
203
done
clear
C)
279
done
clear
D)
101.5
done
clear
View Answer play_arrow
-
question_answer55) If \[\Delta E\] is the heat of reaction for \[{{C}_{2}}{{H}_{5}}OH(l)+3{{O}_{2}}(g)\xrightarrow{{}}2C{{O}_{2}}(g)+3{{H}_{2}}O(l)\] at constant volume, the \[\Delta H\] (heat of reaction at constant pressure) at constant temperature is:
A)
\[\Delta H=\Delta E+RT\]
done
clear
B)
\[\Delta H=\Delta E-RT\]
done
clear
C)
\[\Delta H=\Delta E-2RT\]
done
clear
D)
\[\Delta H=\Delta E+2RT\]
done
clear
View Answer play_arrow
-
question_answer56) For a reversible reaction, if the concentrations of the reactants are doubled, the equilibrium constant will be:
A)
one-fourth
done
clear
B)
halved
done
clear
C)
doubled
done
clear
D)
the same
done
clear
View Answer play_arrow
-
question_answer57) The entropy change in the fusion of one mole of a solid melting at \[{{27}^{o}}C\] (Latent heat of fusion, \[2930\,\,J\,mo{{l}^{-1}}\]) is:
A)
\[9.77\,\,J\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
B)
\[10.73\,\,J\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
C)
\[2930\,\,J\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
D)
\[108.5\,\,J\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer58) The half-life of a radioactive isotope is three hours. If the initial mass of the isotope was 300g, the mass which remained undecayed after 18 hours would be:
A)
4.68 g
done
clear
B)
2.34 g
done
clear
C)
1.17 g
done
clear
D)
9.36 g
done
clear
View Answer play_arrow
-
question_answer59) Cell reaction is spontaneous when:
A)
\[E_{red}^{o}\] is negative
done
clear
B)
\[E_{red}^{o}\] is positive
done
clear
C)
\[\Delta {{G}^{o}}\] is negative
done
clear
D)
\[\Delta {{G}^{o}}\]is positive
done
clear
View Answer play_arrow
-
question_answer60) \[C{{u}^{+}}(aq)\] is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction: \[2C{{u}^{+}}(aq)C{{u}^{2+}}(aq)+Cu(s)\] choose correct \[{{E}^{o}}\] for above reaction if \[E_{C{{u}^{2+}}/Cu}^{0}=0.34\,V\] and \[E_{C{{u}^{2+}}/C{{u}^{+}}}^{0}=0.15\,V\]
A)
- 0.38 V
done
clear
B)
+ 0.49 V
done
clear
C)
+ 0.38 V
done
clear
D)
- 0.19 V
done
clear
View Answer play_arrow
-
question_answer61) Which of the following expressions correctly represents the relationship between the average molar kinetic energy, \[\overline{KE}\], of CO and \[{{N}_{2}}\] molecules at the same temperature ?
A)
\[{{\overline{KE}}_{CO}}\,<{{\overline{KE}}_{{{N}_{2}}}}\]
done
clear
B)
\[{{\overline{KE}}_{CO}}\,>{{\overline{KE}}_{{{N}_{2}}}}\]
done
clear
C)
\[{{\overline{KE}}_{CO}}={{\overline{KE}}_{{{N}_{2}}}}\]
done
clear
D)
Cannot be predicted unless volumes of die gases are given
done
clear
View Answer play_arrow
-
question_answer62) Which of the following statements about pH and \[{{H}^{+}}\] ion concentration is incorrect?
A)
Addition of one drop of concentrated HCl in \[N{{H}_{4}}OH\] solution decreases pH of the solution
done
clear
B)
A solution of the mixture of one equivalent of each of \[C{{H}_{3}}COOH\] and NaOH has a pH of 7
done
clear
C)
pH of pure neutral water is not zero
done
clear
D)
A cold and cone. \[{{H}_{2}}S{{O}_{4}}\] has lower \[{{H}^{+}}\] ion concentration than a dilute solution of \[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer63) Which one of the following is true for any diprotic acid, \[{{H}_{2}}X\]?
A)
\[{{K}_{{{a}_{2}}}}={{K}_{{{a}_{1}}}}\]
done
clear
B)
\[{{K}_{{{a}_{2}}}}>{{K}_{{{a}_{1}}}}\]
done
clear
C)
\[{{K}_{{{a}_{2}}}}<{{K}_{{{a}_{1}}}}\]
done
clear
D)
\[{{K}_{{{a}_{2}}}}=\frac{1}{K{{a}_{1}}}\]
done
clear
View Answer play_arrow
-
question_answer64) Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
HCl
done
clear
D)
HF
done
clear
View Answer play_arrow
-
question_answer65) In the following reaction, how is the rate of appearance of the underlined product related to the rate of disappearance of the underlined reactant? \[BrO_{3}^{-}(aq)+\underline{5B{{r}^{-}}(aq)}+6{{H}^{+}}(aq)\xrightarrow{{}}\underline{3B{{r}_{2}}(l)}+3{{H}_{2}}O(l)\]
A)
\[\frac{d[B{{r}_{2}}]}{dt}=-\frac{5}{3}\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
B)
\[\frac{d[B{{r}_{2}}]}{dt}=-\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
C)
\[\frac{d[B{{r}_{2}}]}{dt}=\frac{3}{5}\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
D)
\[\frac{d[B{{r}_{2}}]}{dt}=-\frac{3}{5}\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
View Answer play_arrow
-
question_answer66) For the equilibrium \[MgC{{O}_{3}}MgO(s)+C{{O}_{2}}(g)\] Which of the following expressions is correct?
A)
\[{{K}_{p}}={{P}_{C{{O}_{2}}}}\]
done
clear
B)
\[{{K}_{p}}=\frac{[MgO]\,[C{{O}_{2}}]}{[MgC{{O}_{3}}]}\]
done
clear
C)
\[{{K}_{p}}=\frac{{{P}_{MgO}}+{{P}_{C{{O}_{2}}}}}{{{P}_{MgC{{O}_{3}}}}}\]
done
clear
D)
\[{{K}_{p}}=\frac{{{P}_{MgO}}+{{P}_{C{{O}_{2}}}}}{{{P}_{MgC{{O}_{3}}}}}\]
done
clear
View Answer play_arrow
-
question_answer67) Among the following the electron deficient compound is:
A)
\[BC{{l}_{3}}\]
done
clear
B)
\[CC{{l}_{4}}\]
done
clear
C)
\[PC{{l}_{5}}\]
done
clear
D)
\[BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer68) The method usually employed for the precipitation of a colloidal solution is:
A)
dialysis
done
clear
B)
addition of electrolytes
done
clear
C)
diffusion through animal membrane
done
clear
D)
condensation
done
clear
View Answer play_arrow
-
question_answer69) The relationship between the dissociation energy of N2 and \[N_{2}^{+}\] is:
A)
dissociation energy of \[N_{2}^{+}\] > dissociation energy of \[N_{2}^{+}\]
done
clear
B)
dissociation energy of \[{{N}_{2}}=\] dissociation energy of \[N_{2}^{+}\]
done
clear
C)
dissociation energy of \[{{N}_{2}}>\] dissociation energy of \[N_{2}^{+}\]
done
clear
D)
dissociation energy of \[{{N}_{2}}\] can either be lower or higher than the dissociation energy of \[N_{2}^{+}\]
done
clear
View Answer play_arrow
-
question_answer70) The factor of \[\Delta G\] values is important in metallurgy. The \[\Delta G\] values for the following reactions at 800°C are given as: \[\begin{align} & {{S}_{2}}(s)+2{{O}_{2}}(g)\to 2S{{O}_{2}}(g)\,;\,\Delta G=-544\,kJ \\ & 2Zn(s)+{{S}_{2}}O\to \,2ZnS(s)\,;\Delta G=-293kJ \\ & 2Zn(s)+{{O}_{2}}(g)\to 2ZnO(s);\Delta G=-480\,kJ \\ \end{align}\] Then \[\Delta G\] for the reaction \[2ZnS(s)+3{{O}_{2}}(g)\xrightarrow{{}}2ZnO(s)+2S{{O}_{2}}(g)\] will be:
A)
-357 kJ
done
clear
B)
-731 kJ
done
clear
C)
-773 kJ
done
clear
D)
-229 kJ
done
clear
View Answer play_arrow
-
question_answer71) Which one of the following is not paramagnetic?
A)
NO
done
clear
B)
\[N_{2}^{+}\]
done
clear
C)
CO
done
clear
D)
\[O_{2}^{-}\]
done
clear
View Answer play_arrow
-
question_answer72) Among the following ions the \[p\pi -d\pi \] overlap could be present in:
A)
\[NO_{2}^{-}\]
done
clear
B)
\[NO_{3}^{-}\]
done
clear
C)
\[PO_{4}^{3-}\]
done
clear
D)
\[CO_{3}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer73) A compound formed by elements A and B crystallises in the cubic structure where A atoms are at the comers of a cube and B atoms are at the face centres. The formula of die compound is:
A)
\[A & {{ & }_{2}}{{B}_{2}}\]
done
clear
B)
\[A{{B}_{3}}\]
done
clear
C)
AB
done
clear
D)
\[{{A}_{3}}B\]
done
clear
View Answer play_arrow
-
question_answer74) Assuming fully decomposed, the volume of \[C{{O}_{2}}\] released at STP on heating 9.85g of \[BaC{{O}_{3}}\] (Atomic mass, Ba = 137) will be:
A)
1.12 L
done
clear
B)
0.84 L
done
clear
C)
2.24 L
done
clear
D)
4.06 L
done
clear
View Answer play_arrow
-
question_answer75) A compound contains atoms of three elements A, B and C. If the oxidation number of A is +2, B is +5, and that of C is -2, the possible formula of the compound is:
A)
\[{{A}_{2}}{{(B{{C}_{3}})}^{2}}\]
done
clear
B)
\[{{A}_{3}}{{(B{{C}_{4}})}^{2}}\]
done
clear
C)
\[{{A}_{3}}{{({{B}_{4}}C)}_{2}}\]
done
clear
D)
\[AB{{C}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer76) The correct structure of \[Fe{{(CO)}_{5}}\] is:
A)
trigonal bipyramidal
done
clear
B)
octahedral
done
clear
C)
tetrahedral
done
clear
D)
square pyramidal
done
clear
View Answer play_arrow
-
question_answer77) Which one of the following complexes will have four different isomers?
A)
\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
done
clear
B)
\[[Co(en)(N{{H}_{3}})C{{l}_{2}}]Cl\]
done
clear
C)
\[[Co{{(PP{{H}_{3}})}_{2}}(N{{H}_{3}})C{{l}_{2}}]Cl\]
done
clear
D)
\[[Co{{(en)}_{3}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer78) Which one of the following forms a colourless solution in aqueous medium? (Atomic number: Sc = 21, Ti = 22, V = 23, Cr = 24)
A)
\[{{V}^{3+}}\]
done
clear
B)
\[C{{r}^{3+}}\]
done
clear
C)
\[T{{i}^{3+}}\]
done
clear
D)
\[S{{c}^{3+}}\]
done
clear
View Answer play_arrow
-
question_answer79) Among die following groupings which represents the collection of isoelectronic species?
A)
\[NO,\,C{{N}^{-}},\,{{N}_{2}},\,O_{2}^{-}\]
done
clear
B)
\[N{{O}^{+}},\,C_{2}^{2-},\,O_{2}^{-},\,CO\]
done
clear
C)
\[{{N}_{2}},\,C_{2}^{2-},\,CO,\,NO\]
done
clear
D)
\[CO,\,N{{O}^{+}},\,C{{N}^{-}},\,C_{2}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer80) In the separation of \[C{{u}^{2+}}\] and \[C{{d}^{2+}}\] in 2nd group of qualitative analysis of cations, tetramine copper (II) sulphate and tetrammine cadmium (II) sulphate react with KCN to form the corresponding cyano complexes, which one of the following pairs of the complexes and their relative stability enables the separation of \[C{{u}^{2+}}\] and \[C{{d}^{2+}}\]?
A)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\] : less stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : more stable
done
clear
B)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\] : more stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : less stable
done
clear
C)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\] : less stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : more stable
done
clear
D)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\] : more stable and \[{{K}_{2}}[Cd{{(CN)}_{4}}]\] : less stable
done
clear
View Answer play_arrow
-
question_answer81) Of the following transition metals, the maximum numbers of oxidation states are exhibited by:
A)
chromium (Z = 24)
done
clear
B)
manganese (Z = 25)
done
clear
C)
iron (Z = 26)
done
clear
D)
titanium (Z = 22)
done
clear
View Answer play_arrow
-
question_answer82) Which one of the following has magnesium?
A)
Vitamin \[{{B}_{12}}\]
done
clear
B)
Chlorophyll
done
clear
C)
Haemocyanin
done
clear
D)
Carbonic anhydrase
done
clear
View Answer play_arrow
-
question_answer83) Propan-1-ol may be prepared by reaction of propene with:
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-O-O-H\]
done
clear
B)
\[{{H}_{3}}B{{O}_{3}}\]
done
clear
C)
\[{{B}_{2}}{{H}_{6}}/NaOH-{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}{{O}_{4}}/{{H}_{2}}O\]
done
clear
View Answer play_arrow
-
question_answer84) During reduction of aldehydes with hydrazine and potassium hydroxide, the first is the formation of:
A)
\[R-CH=N-N{{H}_{2}}\]
done
clear
B)
\[R-C\equiv \equiv N\]
done
clear
C)
\[R-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-N{{H}_{2}}\]
done
clear
D)
\[R-CH=NH\]
done
clear
View Answer play_arrow
-
question_answer85) In Friedel-Craft?s synthesis of toluene, the reactants in addition to anhydrous \[AlC{{l}_{3}}\] are:
A)
\[{{C}_{6}}{{H}_{5}}Cl+C{{H}_{4}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl+C{{H}_{3}}Cl\]
done
clear
C)
\[{{C}_{6}}{{H}_{6}}+C{{H}_{4}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
-
question_answer86) \[\alpha \]-D(+) - glucose and \[\beta \]-D(+)-glucose are:
A)
anomers
done
clear
B)
epimers
done
clear
C)
enantiomers
done
clear
D)
geometrical isomers
done
clear
View Answer play_arrow
-
question_answer87) But-2-ene exhibits cu-trans isomerism due to:
A)
rotation around \[{{C}_{2}}-{{C}_{3}}\] double bond
done
clear
B)
rotation around \[{{C}_{3}}-{{C}_{4}}\] sigma bond
done
clear
C)
rotation around \[{{C}_{1}}-{{C}_{2}}\] bond
done
clear
D)
restricted rotation around > C = C < bond
done
clear
View Answer play_arrow
-
question_answer88) Reduction by \[LiAl{{H}_{4}}\] of hydrolysed product of an ester gives:
A)
two acids
done
clear
B)
two aldehydes
done
clear
C)
one molecule of alcohol and another of carboxylic acid
done
clear
D)
two alcohols
done
clear
View Answer play_arrow
-
question_answer89) Polarization of electrons in acrolein may be written as:
A)
\[\overset{\delta +}{\mathop{C}}\,{{H}_{2}}=CH-CH=\overset{\delta -}{\mathop{O}}\,\]
done
clear
B)
\[\overset{\delta +}{\mathop{C}}\,{{H}_{2}}=\overset{\delta +}{\mathop{CH}}\,-CH=\overset{{}}{\mathop{O}}\,\]
done
clear
C)
\[\overset{\delta +}{\mathop{C}}\,{{H}_{2}}=\overset{{}}{\mathop{CH}}\,-CH=\overset{\delta +}{\mathop{O}}\,\]
done
clear
D)
\[\overset{\delta +}{\mathop{C}}\,{{H}_{2}}=\overset{{}}{\mathop{CH}}\,-\overset{\delta +\,\,}{\mathop{CH}}\,=\overset{{}}{\mathop{O}}\,\]
done
clear
View Answer play_arrow
-
question_answer90) An organic compound A on reduction gives compound B which on reaction with chloroform and potassium hydroxide forms C. The compound C on catalytic reduction gives N-methylaniline. The compound A is:
A)
nitrobenzene
done
clear
B)
nitromethane
done
clear
C)
methylamine
done
clear
D)
aniline
done
clear
View Answer play_arrow
-
question_answer91) Among the following alkenes, 1-butene cis-2-butene trans-2-2butene I II III the decreasing order of stability is:
A)
II > I > III
done
clear
B)
III > II > I
done
clear
C)
III > I > II
done
clear
D)
I > II > III
done
clear
View Answer play_arrow
-
question_answer92) The dihedral angle between two C?H bonds in the staggered conformation of ethane is:
A)
\[{{60}^{\text{o}}}\]
done
clear
B)
\[{{180}^{\text{o}}}\]
done
clear
C)
\[{{0}^{\text{o}}}\]
done
clear
D)
\[{{120}^{\text{o}}}\]
done
clear
View Answer play_arrow
-
question_answer93) The ionization constant of phenol is higher than that of ethanol because:
A)
phenoxide ion is bulkier than ethoxide
done
clear
B)
phenoxide ion is stronger base than ethoxide
done
clear
C)
phenoxide ion is stabilized through derealization
done
clear
D)
phenoxide ion is less stable than ethoxide
done
clear
View Answer play_arrow
-
question_answer94) Which one of the following arrangements does not truly represent the property indicated against it?
A)
\[B{{r}_{2}}<C{{l}_{2}}<{{F}_{2}}\] : Oxidising power
done
clear
B)
\[B{{r}_{2}}<C{{l}_{2}}<{{F}_{2}}\] : Electronegativity
done
clear
C)
\[B{{r}_{2}}<C{{l}_{2}}<{{F}_{2}}\] : Electron affinity
done
clear
D)
\[B{{r}_{2}}<C{{l}_{2}}<{{F}_{2}}\] : Bond energy
done
clear
View Answer play_arrow
-
question_answer95) Among the following compounds the decreasing order of reactivity towards electrophilic substitution is :
A)
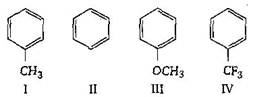
II > I > III > IV
done
clear
B)
III > I > II > IV
done
clear
C)
IV > I > II > III
done
clear
D)
I > II > III > IV
done
clear
View Answer play_arrow
-
question_answer96) The (R) - and (S) - enantiomers of an optically active compound differ in:
A)
their solubility in a chiral solvents
done
clear
B)
their reactivity with a chiral reagents
done
clear
C)
their optical rotation of plane polarised light
done
clear
D)
their melting points
done
clear
View Answer play_arrow
-
question_answer97) Benzoic acid may be converted to ethyl benzoate by reaction with:
A)
sodium ethoxide
done
clear
B)
ethyl chloride
done
clear
C)
dry \[HCl-{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
ethanol
done
clear
View Answer play_arrow
-
question_answer98) \[C{{F}_{2}}=C{{F}_{2}}\] is a monomer of:
A)
buna-S
done
clear
B)
Teflon
done
clear
C)
glyptal
done
clear
D)
nylon-6
done
clear
View Answer play_arrow
-
question_answer99) The hormone which controls the processes like burning of fats, proteins and carbohydrates to liberate energy in the body is:
A)
cortisone
done
clear
B)
thyroxine
done
clear
C)
adrenaline
done
clear
D)
insulin
done
clear
View Answer play_arrow
-
question_answer100) Which of the following colligative property can provide molar mass of proteins (or polymers or colloids) with greatest precision?
A)
Osmotic pressure
done
clear
B)
Elevation of boiling point
done
clear
C)
Depression of freezing point
done
clear
D)
Relative lowering of vapour pressure
done
clear
View Answer play_arrow
-
question_answer101) Darwin's finches provide an excellent evidence in favour of organic evolution. These are related to which of the following evidences?
A)
Embryology
done
clear
B)
Palaeontology (or fossils)
done
clear
C)
Anatomy
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer102) Which one of the following is correctly matched pair of a certain plant family and its one example?
A)
Malvaceae?Cotton
done
clear
B)
Leguminosae?Mango (or Sunflower)
done
clear
C)
Cucurbitaceae?Orange
done
clear
D)
Brassicaceae?Wheat
done
clear
View Answer play_arrow
-
question_answer103) Relative Biological Effectiveness (RBE) usually refers to the damages caused by:
A)
low temperature
done
clear
B)
high temperature
done
clear
C)
radiation
done
clear
D)
pollution
done
clear
View Answer play_arrow
-
question_answer104) The greatest biomass of autotrophs in the world's oceans is that of:
A)
benthic brown algae, coastal fed algae and daphnids
done
clear
B)
benthic diatoms and marine viruses
done
clear
C)
sea grasses and slime molds
done
clear
D)
free-floating micro-algae, cyanobacteria and nanoplankton
done
clear
View Answer play_arrow
-
question_answer105) Enzymes are absent in:
A)
algae
done
clear
B)
fungi
done
clear
C)
cyanobacteria
done
clear
D)
viruses
done
clear
View Answer play_arrow
-
question_answer106) An institution where valuable plant material-likely to become irretrievably lost in the wild or in cultivation-is preserved in a viable condition is known as:
A)
genome
done
clear
B)
gene library
done
clear
C)
gene bank
done
clear
D)
herbarium
done
clear
View Answer play_arrow
-
question_answer107) For yielding one molecule of glucose, the Calvin cycle turns:
A)
two times
done
clear
B)
four times
done
clear
C)
six times
done
clear
D)
eight times
done
clear
View Answer play_arrow
-
question_answer108) Photochemical reactions in the chloroplast are directly involved in:
A)
photolysis of water and phosphorylation of ADP to ATP
done
clear
B)
formation of phosphoglyceric acid
done
clear
C)
synthesis of glucose and starch
done
clear
D)
fixation of carbon dioxide
done
clear
View Answer play_arrow
-
question_answer109) The first (initiating) step in photosynthesis is:
A)
ionisation of water
done
clear
B)
attachment of \[C{{O}_{2}}\] to 5-carbon sugar
done
clear
C)
excitation of chlorophyll by a photon of light
done
clear
D)
formation of ATP
done
clear
View Answer play_arrow
-
question_answer110) In most fungi, the food material is stored in the form of:
A)
starch
done
clear
B)
glucose
done
clear
C)
sucrose
done
clear
D)
glycogen
done
clear
View Answer play_arrow
-
question_answer111) Feed back inhibition of an enzymatic reaction is caused by:
A)
substrate
done
clear
B)
enzyme
done
clear
C)
end product
done
clear
D)
rise in temperature
done
clear
View Answer play_arrow
-
question_answer112) Small proteins produced by vertebrate cells naturally in response to viral infections and which inhibit mutliplication of viruses are called:
A)
immunoglobulins
done
clear
B)
interferons
done
clear
C)
antitoxins
done
clear
D)
lipoproteins
done
clear
View Answer play_arrow
-
question_answer113) Which one of the following statements is correct with reference to honey bees?
A)
Bees wax is a waste (excretory) product of honey bees
done
clear
B)
Communication among honey bees was discovered by Von Frisch
done
clear
C)
Apis indica is largest wild bee in India
done
clear
D)
Honey is predominantly sucrose and arabinose
done
clear
View Answer play_arrow
-
question_answer114) Lysosomes are the reservoirs (store houses) of:
A)
hydrolytic enzymes
done
clear
B)
secretory glycoproteins
done
clear
C)
RNA and protein
done
clear
D)
fats (or sugars or ATP)
done
clear
View Answer play_arrow
-
question_answer115) Black rust of wheat is caused by a member species of the genus:
A)
Mucor
done
clear
B)
Rhizopus
done
clear
C)
Aspergillus
done
clear
D)
Puccinia
done
clear
View Answer play_arrow
-
question_answer116) The first successfully cloned mammals (animal) that gained worldwide publicity was:
A)
Molly (a sheep)
done
clear
B)
Polly (a sheep)
done
clear
C)
Chance (a bull)
done
clear
D)
Dolly (a sheep)
done
clear
View Answer play_arrow
-
question_answer117) As per geological time scale, hominids evolved during:
A)
Miocene
done
clear
B)
Pliocene
done
clear
C)
Pleistocene
done
clear
D)
Oligocene
done
clear
View Answer play_arrow
-
question_answer118) Plasmids are suitable vectors for gene cloning because:
A)
these are small circular DNA molecules which can integrate with host chromosomal DNA
done
clear
B)
these are small circular DNA molecules with their own replication origin site
done
clear
C)
these can shuttle between prokaryotic and eukaryotic cells
done
clear
D)
these often carry antibiotic resistance genes
done
clear
View Answer play_arrow
-
question_answer119) A bacterium which has found extensive use in genetic engineering work in plants is:
A)
Bacillus coagulans
done
clear
B)
Clostridium septicum
done
clear
C)
Xanthomohas citric for Pseudomonas)
done
clear
D)
Agrobacterium tumefaciens
done
clear
View Answer play_arrow
-
question_answer120) Enzymes enhance the rate of a reaction by:
A)
lowering the activation energy of the reaction
done
clear
B)
combining with the product as soon as it is formed
done
clear
C)
forming a reactant-product complex
done
clear
D)
changing the equilibrium point of the reaction
done
clear
View Answer play_arrow
-
question_answer121) One of the most important reasons why wild plants should thrive is that these are good sources of:
A)
unsaturated edible oils
done
clear
B)
highly nutritive animals feed
done
clear
C)
genes for resistance to diseases and pests
done
clear
D)
rare and highly sought after fruits of medical importance
done
clear
View Answer play_arrow
-
question_answer122) Producing a giant mouse in the laboratory was possible through:
A)
gene mutation
done
clear
B)
gene manipulation
done
clear
C)
gene synthesis
done
clear
D)
gene duplication
done
clear
View Answer play_arrow
-
question_answer123) Industrial production of ethanol from starch is brought about by a certain species of:
A)
Azotobacter
done
clear
B)
Lactobacillus
done
clear
C)
Saccharomyces
done
clear
D)
Penicillim
done
clear
View Answer play_arrow
-
question_answer124) A water fem which is used as a green manure in rice fields is:
A)
Salvinia
done
clear
B)
Mucor
done
clear
C)
Aspergillus
done
clear
D)
Azolla
done
clear
View Answer play_arrow
-
question_answer125) The endangered largest living lemur Idri idri is inhabitant of:
A)
Madagascar
done
clear
B)
Mauritius
done
clear
C)
Sri Lanka
done
clear
D)
India
done
clear
View Answer play_arrow
-
question_answer126)
ATP is a: nucleotide *-correct-answer-description-* A nucleotide contains (a) a S-C sugar, (b) a phosphate molecule and (c) a nitrogenous base. ATP is also a nucleotide. It also has a 5-C sugar (ribose), 3 phosphate molecules and a nitrogenous base (adenine). 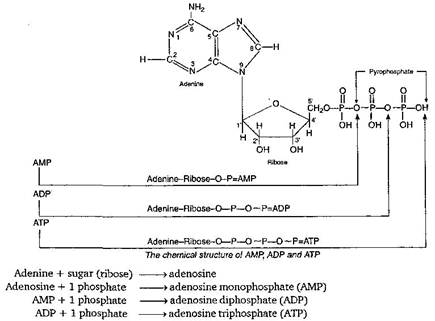
A)
done
clear
B)
nucleosome
done
clear
C)
purine base
done
clear
D)
nucleoside
done
clear
View Answer play_arrow
-
question_answer127) The largest known ovules, largest male and female gametes, and largest plants are found among:
A)
tree ferns and some monocots
done
clear
B)
angiosperms
done
clear
C)
gymnosperms
done
clear
D)
dicotyledonous plants
done
clear
View Answer play_arrow
-
question_answer128) Which one of the following correctly matches a Sexually Transmitted Disease (STD) with its pathogen?
A)
AIDS?Bacillus anthracis
done
clear
B)
Syphilis?Treponema pallidum
done
clear
C)
Urethritis?Entamoeba gingivalis
done
clear
D)
Gonorrhoea?Leishmania donovani
done
clear
View Answer play_arrow
-
question_answer129) Removal of apical (terminal) bud of a flowering plant (or pruning of a flowering plant) leads to:
A)
formation of new apical buds
done
clear
B)
formation of adventitious roots on the cut side
done
clear
C)
early flowering (or stopping of floral growth)
done
clear
D)
promotion of lateral branches
done
clear
View Answer play_arrow
-
question_answer130) The cell organelle involved in the glycosylation of proteins is:
A)
ribosome
done
clear
B)
peroxisome
done
clear
C)
mitochondria
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
-
question_answer131) Among mammals, a significant role is the digestion of milk is played by:
A)
rennin
done
clear
B)
invertase
done
clear
C)
amylase
done
clear
D)
intestinal bacteria
done
clear
View Answer play_arrow
-
question_answer132) Which one of the following is not a vestigial part in human?
A)
Coccyx
done
clear
B)
Fingernails
done
clear
C)
Third molar of each side in each jaw
done
clear
D)
Segmental muscles of abdomen
done
clear
View Answer play_arrow
-
question_answer133) Conjugated proteins containing carbohydrates as prosthetic group are called:
A)
lipoproteins
done
clear
B)
nucleoproteins
done
clear
C)
glycoproteins
done
clear
D)
chromoproteins
done
clear
View Answer play_arrow
-
question_answer134) Which one of the following is correctly matched pair of the given secretion and its primary role in human physiology?
A)
Sebum?Sexual attraction
done
clear
B)
Sweat?Thermoregulation
done
clear
C)
Saliva?Tasting food
done
clear
D)
Tears?Excretion of salts
done
clear
View Answer play_arrow
-
question_answer135) The function of copper T is to prevent:
A)
fertilization
done
clear
B)
egg maturation
done
clear
C)
ovulation
done
clear
D)
implantation of blastocyst
done
clear
View Answer play_arrow
-
question_answer136) The replication of DNA is a pre-requisite for a eukaryotic cell to undergo division. During the cell cycle the DNA replicates in:
A)
S phase
done
clear
B)
\[{{G}_{1}}\] phase
done
clear
C)
\[{{G}_{2}}\] phase
done
clear
D)
M phase
done
clear
View Answer play_arrow
-
question_answer137) Which one of the following characters studied by Mendel in garden pea was found to be dominant?
A)
Green seed colour
done
clear
B)
Terminal flower position
done
clear
C)
Green pod colour
done
clear
D)
Wrinkled seed
done
clear
View Answer play_arrow
-
question_answer138) Geocarpic fruits are produced by:
A)
carrot
done
clear
B)
onion
done
clear
C)
ground nut
done
clear
D)
watermelon
done
clear
View Answer play_arrow
-
question_answer139) Special kinds of roots called pneumatophores are characteristics of the plants growing in:
A)
sandy soils
done
clear
B)
saline soils
done
clear
C)
marshy places and salt lakes
done
clear
D)
dryland regions
done
clear
View Answer play_arrow
-
question_answer140) The plants growing in magnesium-deficient soil, but sprayed with urea would show:
A)
deep green foliage
done
clear
B)
loss of pigments in petals
done
clear
C)
early flowering
done
clear
D)
yellowing of leaves
done
clear
View Answer play_arrow
-
question_answer141) Coconut milk (coconut water) is widely used in tissue culture because it contains:
A)
auxins
done
clear
B)
ethylene
done
clear
C)
cytokinin
done
clear
D)
gibbereliins
done
clear
View Answer play_arrow
-
question_answer142) A research scholar once collected certain alga and found that its cells contained chlorophyll a, chlorophyll d and phycoerythrin. The alga must belong to:
A)
Chlorophyceae
done
clear
B)
Rhodopyceae
done
clear
C)
Bacillariophyceae
done
clear
D)
Cyanophyceae
done
clear
View Answer play_arrow
-
question_answer143) Fixation of one molecule of \[C{{O}_{2}}\] through Calvin cycle requires:
A)
3 ATP and 3 \[NADP{{H}_{2}}\] molecules
done
clear
B)
3 ATP and 2 \[NADP{{H}_{2}}\] molecules
done
clear
C)
2 ATP and 1 \[NADP{{H}_{2}}\] molecules
done
clear
D)
1 ATP and 2 \[NADP{{H}_{2}}\] molecules
done
clear
View Answer play_arrow
-
question_answer144) In the life history of ferns, meiosis occurs at the time of:
A)
formation of spores
done
clear
B)
formation of antheridia and archegonia
done
clear
C)
formation of gametes
done
clear
D)
germination of spores
done
clear
View Answer play_arrow
-
question_answer145) In a given plant, red colour (R) of fruits is dominant over white fruit (r); and tallness (T) is dominant over dwarfness (t). If a plant with genotype RRTt is crossed with a plant of genotype rrtt, what will be the percentage of tall plants with red fruits in the next generation?
A)
100%
done
clear
B)
25%
done
clear
C)
50%
done
clear
D)
75%
done
clear
View Answer play_arrow
-
question_answer146) Mutation generally produces:
A)
recessive genes
done
clear
B)
lethal genes
done
clear
C)
polygenes
done
clear
D)
dominant genes
done
clear
View Answer play_arrow
-
question_answer147) The triplet codon in m-RNA which is usually the starting point for protein synthesis, is:
A)
AUU
done
clear
B)
AUG
done
clear
C)
AUA
done
clear
D)
AUC
done
clear
View Answer play_arrow
-
question_answer148) Zinc as a nutrient is used by the plants in m form of:
A)
Zn
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
ZnO
done
clear
D)
\[ZnS{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer149) In an animal cell, protein synthesis takes place:
A)
only on the ribosomes present in the cytosol
done
clear
B)
only on the ribosomes attached to nucelar envelope and endoplasmic reticulum
done
clear
C)
on ribosomes present in the nucleolus as well as in cytoplasm
done
clear
D)
on ribosomes present in the cytosol as well as in the mitochondria
done
clear
View Answer play_arrow
-
question_answer150) The most important feature of all living systems from the point of view of their continuity is their capacity to:
A)
produce gametes
done
clear
B)
utilise oxygen to generate energy
done
clear
C)
use solar energy for metabolic activities
done
clear
D)
replicate the genetic information
done
clear
View Answer play_arrow
-
question_answer151) Many scientists consider viruses as living entities because these:
A)
respire
done
clear
B)
can cause diseases
done
clear
C)
reproduce (inside host)
done
clear
D)
respond to tough environment
done
clear
View Answer play_arrow
-
question_answer152) RNA and DNA are similar in:
A)
having similar sugars
done
clear
B)
having similar pyrimidine bases
done
clear
C)
being capable to replicate
done
clear
D)
being polymers of nucleotides
done
clear
View Answer play_arrow
-
question_answer153) In three-dimensional view, a transfer RNA (r-RNA) molecule appears:
A)
Y-shaped
done
clear
B)
S-shaped
done
clear
C)
E-shaped
done
clear
D)
L-shaped
done
clear
View Answer play_arrow
-
question_answer154) Anticodon is present on:
A)
r-RNA
done
clear
B)
t-RNA
done
clear
C)
mt-DNA
done
clear
D)
m-RNA
done
clear
View Answer play_arrow
-
question_answer155) Extranuclear DNA (genes) are located in:
A)
lysosomes and chloroplasts
done
clear
B)
Golgi complex and ribosomes
done
clear
C)
chloroplasts and mitochondria
done
clear
D)
ribosomes and mitochondria
done
clear
View Answer play_arrow
-
question_answer156) During replication of DNA, its two strands separate. Each of these serves as a template for the formation of new strands. Such type of replication is called:
A)
non-conservative
done
clear
B)
semi-conservative
done
clear
C)
flexible
done
clear
D)
conservative.
done
clear
View Answer play_arrow
-
question_answer157) The length of one turn of the helix in a B-form DNA is approximately:
A)
0.34 nm
done
clear
B)
20 nm
done
clear
C)
2 nm
done
clear
D)
3.4 nm
done
clear
View Answer play_arrow
-
question_answer158) During ceil division, the spindle fibres get attached to the condensing chromosome at a highly differentiated region. This region is called:
A)
chromomere
done
clear
B)
chromocentre
done
clear
C)
centriole
done
clear
D)
kinetochore
done
clear
View Answer play_arrow
-
question_answer159) Mongoloid idiocy in human beings caused by me trisomy of chromosome 21 is also known as:
A)
Down's syndrome
done
clear
B)
Turner's syndrome
done
clear
C)
Klinefelter's syndrome
done
clear
D)
Tay-Sachs disease
done
clear
View Answer play_arrow
-
question_answer160) Drosophila flies with XXY genotype are females, but human beings with such genotype are abnormal males. It shows that:
A)
Y-chromosome is essential for sex determination in Drosophila
done
clear
B)
Y-chromosome is female determinating in Drosophila
done
clear
C)
Y-chromosome is male determining in human beings
done
clear
D)
Y-chromosome has no role in sex determination either in Drosophila or in human beings
done
clear
View Answer play_arrow
-
question_answer161) Which one pair of parents out of the following is most likely get a child who would suffer from hemolytic disease of the new-born:
A)
\[R{{h}^{+}}\] mother and \[R{{h}^{-}}\] father
done
clear
B)
\[R{{h}^{-}}\] mother and \[R{{h}^{-}}\] father
done
clear
C)
\[R{{h}^{+}}\] mother and \[R{{h}^{+}}\] father
done
clear
D)
\[R{{h}^{-}}\] mother and \[R{{h}^{+}}\] father
done
clear
View Answer play_arrow
-
question_answer162) During organ differentiation in Drosophila, an organ is modified to another organ (such as wings may be replaced by legs). Genes responsible for such metamorphosis are called:
A)
double dominant genes
done
clear
B)
plastid genes
done
clear
C)
complementary genes
done
clear
D)
homeotic genes
done
clear
View Answer play_arrow
-
question_answer163) One function of telomeres in a chromosome is to:
A)
?seal? the ends of the chromosomes
done
clear
B)
help two chromatids to move towards poles
done
clear
C)
starts RNA synthesis
done
clear
D)
identify the correct member of the homologous pair of chromosome
done
clear
View Answer play_arrow
-
question_answer164) Melatonin is secreted by:
A)
skin
done
clear
B)
thymus
done
clear
C)
pituitary
done
clear
D)
pineal gland
done
clear
View Answer play_arrow
-
question_answer165) What is correct regarding leucocytes?
A)
These can squeeze out through (can cross) the capillary walls
done
clear
B)
These are enucleate
done
clear
C)
Sudden fall in their number indicates cancer
done
clear
D)
These are produced in thymus
done
clear
View Answer play_arrow
-
question_answer166) Which one of the following is a skull hone?
A)
Coracoid
done
clear
B)
Arytaenoid
done
clear
C)
Atlas
done
clear
D)
Pterygoid
done
clear
View Answer play_arrow
-
question_answer167) The polysaccharide portion of a peptidoglycan present in the matrix of cartilage is called:
A)
chondriotin
done
clear
B)
ossein
done
clear
C)
cartilagin
done
clear
D)
casein
done
clear
View Answer play_arrow
-
question_answer168) In living beings, ammonia is converted into urea through:
A)
Ornithine cycle
done
clear
B)
citrulline cycle
done
clear
C)
fumarine cycle
done
clear
D)
arginine cycle
done
clear
View Answer play_arrow
-
question_answer169) Which one of the following hormones is involved in the ripening of fruits?
A)
Zeatin
done
clear
B)
Indole acetic acid
done
clear
C)
Ethylene
done
clear
D)
Naphthalene acetic acid
done
clear
View Answer play_arrow
-
question_answer170) Which one of the following primates is believed to be the closest relative of human beings?
A)
Gorilla
done
clear
B)
Rhesus monkey
done
clear
C)
Gibbon
done
clear
D)
Orangutan
done
clear
View Answer play_arrow
-
question_answer171) Which of the following is a correct statement with regard to a certain Mammal and its one feature?
A)
Bat bears feathers
done
clear
B)
Camel has biconcave red blood cells
done
clear
C)
Platypus lays eggs
done
clear
D)
Rat bears cloaca
done
clear
View Answer play_arrow
-
question_answer172) Blastopore is the opening to the exterior of:
A)
coelom
done
clear
B)
coelenterons
done
clear
C)
archentron
done
clear
D)
blastocoels
done
clear
View Answer play_arrow
-
question_answer173) Movement of ions or molecules against the electrochemical gradient is called:
A)
diffusion
done
clear
B)
pinocytosis
done
clear
C)
Brownian movement
done
clear
D)
active transport
done
clear
View Answer play_arrow
-
question_answer174) The enteronephric nephridia of earthworms are mainly concerned with:
A)
digestion
done
clear
B)
respiration
done
clear
C)
osmoregulation
done
clear
D)
excretion of nitrogenous wastes
done
clear
View Answer play_arrow
-
question_answer175) The ability of the vertebrates to produce concentrated (hyperosmotic) urine usually depends upon the:
A)
area of Bowman's capsule epithelium
done
clear
B)
length of Henle's loop
done
clear
C)
length of the proximal convoluted tubule
done
clear
D)
capillary network forming glomerulus
done
clear
View Answer play_arrow
-
question_answer176) The enzyme which catalyses fixation of \[C{{O}_{2}}\] in \[{{C}_{4}}\] plants is:
A)
RuBP carboxylase
done
clear
B)
hydrogenase
done
clear
C)
PEP carboxylase
done
clear
D)
reductase
done
clear
View Answer play_arrow
-
question_answer177) Which of the following is not a characteristic feature of all the chordates?
A)
Presence of coelom
done
clear
B)
Pharyngeal gill clefts in the early embryonic stages
done
clear
C)
A diaphragm that separates thorax from abdomen
done
clear
D)
Dorsal nerve cord
done
clear
View Answer play_arrow
-
question_answer178) Melanocyte Stimulating Hormone (MSH) is produced by:
A)
anterior pituitary
done
clear
B)
posterior pituitary
done
clear
C)
pars intermedia of pituitary
done
clear
D)
parathyroid
done
clear
View Answer play_arrow
-
question_answer179) The feature closely related with the evolution of humans is:
A)
loss of tail
done
clear
B)
flat nails
done
clear
C)
binocular vision
done
clear
D)
shortening of jaw
done
clear
View Answer play_arrow
-
question_answer180) An action potential in the nerve fibre is produced when positive and negative charges on the outside and the inside of the axon membrane are reversed, because:
A)
more potassium ions enter the axon as compared to sodium ions leaving it
done
clear
B)
more sodium ions enter the axon 4 compared to potassium ions leaving it
done
clear
C)
all potassium ions leave the axon
done
clear
D)
all sodium ions enter the axon
done
clear
View Answer play_arrow
-
question_answer181) Numerous filamentous hair-like structures protruding from the tip of a young cob maize are:
A)
anthers
done
clear
B)
hairs
done
clear
C)
hairy projections of the bracts
done
clear
D)
long styles of carpels
done
clear
View Answer play_arrow
-
question_answer182) Which method out of the following renders the seed coat permeable to water so that embryo expansion is not physically retarded?
A)
Stratification
done
clear
B)
Denudation
done
clear
C)
Verbalization
done
clear
D)
Scarification
done
clear
View Answer play_arrow
-
question_answer183) Eight nucleate embryo sacs are:
A)
always bisporic
done
clear
B)
always tetrasporic
done
clear
C)
always monosporic
done
clear
D)
sometimes monosporic, sometimes bisporic and sometimes tetrasporic
done
clear
View Answer play_arrow
-
question_answer184) Double fertilisation (or triple fusion) leading to initiation of endosperm in angiosperms, requires:
A)
fusion of 4 or more polar nuclei and the second male gamete only
done
clear
B)
fusion of 2 polar nuclei and second male gamete only
done
clear
C)
fusion of one polar nucleus and second male gamete only
done
clear
D)
all the above type of fusions in different types of angiosperms
done
clear
View Answer play_arrow
-
question_answer185) Progestrone, which is the most important component of oral contraceptive pills, prevents pregnancy by:
A)
preventing the formation of egg
done
clear
B)
preventing the clevage of the fertilized egg
done
clear
C)
creating unfavourable chemical environment for the sperms to survive in the female reproductive tract
done
clear
D)
blocking ovulation
done
clear
View Answer play_arrow
-
question_answer186) Cleavage in mammalian egg is:
A)
equal holoblastic
done
clear
B)
unequal holoblastic
done
clear
C)
superficial meroblastic
done
clear
D)
discoidal meroblastic
done
clear
View Answer play_arrow
-
question_answer187) Bovine spongiform encephalopathy is a bovine disease. To which of the following human diseases it is related?
A)
Kala azar
done
clear
B)
Encephalitis
done
clear
C)
Cerebral spondylitis
done
clear
D)
Creutzfeldt Jacob disease
done
clear
View Answer play_arrow
-
question_answer188) The type of joint between sternum and ribs in human beings is:
A)
fibrous joint
done
clear
B)
gliding joint
done
clear
C)
angular joint
done
clear
D)
cartilaginous joint
done
clear
View Answer play_arrow
-
question_answer189) What is common between Ascaris lumbricoides and Anopheles stephensi?
A)
Hibernation
done
clear
B)
Metamerism
done
clear
C)
Anaerobic respiration
done
clear
D)
Sexual dimorphism
done
clear
View Answer play_arrow
-
question_answer190) A certain person eats boiled potato; one of the food component in it is:
A)
lactose which is indigestible
done
clear
B)
starch which does not get digested
done
clear
C)
cellulose which is digested by intestinal cellulose
done
clear
D)
DNA which gets digested by pancreatic DNA ase
done
clear
View Answer play_arrow
-
question_answer191) Pulmonary artery is different from pulmonary vein because it has:
A)
larger lumen
done
clear
B)
thick muscular walls
done
clear
C)
no endothelium
done
clear
D)
valves
done
clear
View Answer play_arrow
-
question_answer192) A common scent-producing gland among mammals is:
A)
anal gland
done
clear
B)
prostate gland
done
clear
C)
adrenal gland
done
clear
D)
Bartholin's gland
done
clear
View Answer play_arrow
-
question_answer193) What happens during vascularisation in plants?
A)
Differentiation of procambium followed by primary phloem and then primary xylem
done
clear
B)
Differentiation of procambium, xylem and phloem simultaneously
done
clear
C)
Differentaition of procambium followed immediately by the development of secondary xylem and phloem
done
clear
D)
Differentiation of procambium followed by the development of xylem and phloem
done
clear
View Answer play_arrow
-
question_answer194) A person suffering from the deficiency of the visual pigment rhodopsin is advised to take more of:
A)
radish and potato
done
clear
B)
apple and grapes
done
clear
C)
carrot and ripe papaya
done
clear
D)
guava and ripe banana
done
clear
View Answer play_arrow
-
question_answer195) In a person of advanced age, the hair become thinner gradually. It happens because of decrease in:
A)
synthesis of glucose
done
clear
B)
synthesis of proteins
done
clear
C)
energy availability
done
clear
D)
blood supply
done
clear
View Answer play_arrow
-
question_answer196) In simple epithelium, the cells are:
A)
continuously dividing to provide cells for forming an organ
done
clear
B)
hardened to provide support to the organs
done
clear
C)
loosely connected to one another to from an irregular layer
done
clear
D)
cemented directly to one another to form a single layer
done
clear
View Answer play_arrow
-
question_answer197) Which one of the following amino acids is an essential part of human diet?
A)
Glycine
done
clear
B)
Phenylalanine
done
clear
C)
Serine
done
clear
D)
Aspartic acid
done
clear
View Answer play_arrow
-
question_answer198) Patients suffering from cholera are given a saline drip because:
A)
\[N{{a}^{+}}\] ions help in stopping nerve impulses and hence sensation of pain
done
clear
B)
\[N{{a}^{+}}\] ions help in the retention of water in the body tissues
done
clear
C)
NaCl is an important component of energy supply
done
clear
D)
NaCI furnishes most of the fuel required for cellular activity
done
clear
View Answer play_arrow
-
question_answer199) A piece of bone such as femur of frog, if kept in dilute HCl, for about a week will:
A)
shrink in size
done
clear
B)
assume black colour
done
clear
C)
crack into pieces
done
clear
D)
turn flexible
done
clear
View Answer play_arrow
-
question_answer200) 'Signal hypothesis' for the biosynthesis of secretory type of proteins was proposed by:
A)
Camillo Golgi
done
clear
B)
Blobel and Sabatini
done
clear
C)
Baltimore
done
clear
D)
Sheeler and Bianchi
done
clear
View Answer play_arrow
 \[1500\sqrt{3}\,N\]
\[1500\sqrt{3}\,N\] 



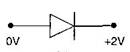
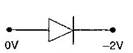
 II > I > III > IV
II > I > III > IV 
