| Figure | Number of lines of symmetry |
| Angle | 1 |
| Scalene triangle | 0 |
| Isosceles triangle | 1 |
| Equilateral triangle | 3 |
| Parallelogram | 0 |
| Rhombus | 2 |
| Isosceles trapezium | 1 |
| Circle | Infinitely Many |
| Semicircle | 1 |
| Regular pentagon | 5 |
| Regular hexagon | 6 |

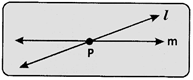
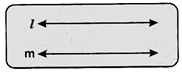
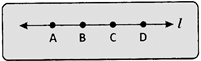 Points that do not lie on the more...
Points that do not lie on the more... 
 1. From the above story what
impression do you get of a patwari in a village?
2. When villagers are in trouble,
who helps them to resolve their problems?
Let us understand the Police and
Patwari and their roles to answer the above questions.
ADMINISTRATION IN A VILLAGE
Two rural government officials
play an important role in administering the villagers during conflicts: the
patwari and the police. The patwari is the land record officer, whose job is to
visit agricultural lands and maintain record of ownership and yield. The local
police solve the quarrels and are in-charge of maintaining law and order in the
village.
THE POLICE
You have seen how the police
helped Ramu, a villager. Villages are divided into areas and put under
different police stations. Each police station is responsible for maintenance
of law and order of more...
1. From the above story what
impression do you get of a patwari in a village?
2. When villagers are in trouble,
who helps them to resolve their problems?
Let us understand the Police and
Patwari and their roles to answer the above questions.
ADMINISTRATION IN A VILLAGE
Two rural government officials
play an important role in administering the villagers during conflicts: the
patwari and the police. The patwari is the land record officer, whose job is to
visit agricultural lands and maintain record of ownership and yield. The local
police solve the quarrels and are in-charge of maintaining law and order in the
village.
THE POLICE
You have seen how the police
helped Ramu, a villager. Villages are divided into areas and put under
different police stations. Each police station is responsible for maintenance
of law and order of more...  In 1947, India became free and
chose the democratic way of governance. Mahatma Gandhi believed that India's
independence must begin from the bottom or at the grass root level. Every
village ought to be responsible for its own affairs and governance. He advocated
Panchayati Raj as the foundation of India's political system.
He called this political more...
In 1947, India became free and
chose the democratic way of governance. Mahatma Gandhi believed that India's
independence must begin from the bottom or at the grass root level. Every
village ought to be responsible for its own affairs and governance. He advocated
Panchayati Raj as the foundation of India's political system.
He called this political more... You need to login to perform this action.
You will be redirected in
3 sec
