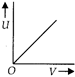
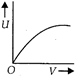
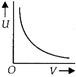
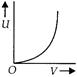
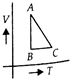
question_answer 1) Which of the following graphs correctly represents the variation of heat energy \[(U)\]produced in a metallic conductor in a given time as a function of potential difference \[\left( V \right)\] across the conductor?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 2) A current of 2 A is passing through a metal wire of cross sectional area \[2\times {{10}^{-6}}{{m}^{2}}\]. If the number density of free electrons in the wire is \[5\times {{10}^{26}}{{m}^{-3}}\], the drift speed of electrons is (given \[e=1.6\times {{10}^{-19}}C\])
A)
\[\frac{1}{16}m{{s}^{-1}}\]
done
clear
B)
\[\frac{1}{40}m{{s}^{-1}}\]
done
clear
C)
\[\frac{1}{80}m{{s}^{-1}}\]
done
clear
D)
\[\frac{1}{32}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 3) Magnetic field at a distance r from an infinitely long straight conductor carrying a steady current varies as
A)
\[\frac{1}{{{r}^{2}}}\]
done
clear
B)
\[\frac{1}{r}\]
done
clear
C)
\[\frac{1}{{{r}^{3}}}\]
done
clear
D)
\[\frac{1}{\sqrt{r}}\]
done
clear
View Answer play_arrow
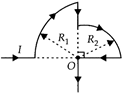
question_answer 4)
In the loop shown, the magnetic induction at the point O is
A)
\[\frac{{{\mu }_{0}}I}{8}\left( \frac{{{R}_{1}}-{{R}_{2}}}{{{R}_{1}}{{R}_{2}}} \right)\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{8}\left( \frac{{{R}_{1}}+{{R}_{2}}}{{{R}_{1}}{{R}_{2}}} \right)\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{8}\left( \frac{{{R}_{1}}{{R}_{2}}}{{{R}_{1}}+{{R}_{2}}} \right)\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 5) An a-particle and a proton moving with the same kinetic energy enter a region of uniform magnetic field at right angles to the field. The ratio of the radii of the paths of a-particle to that of the proton is
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:4\]
done
clear
D)
\[1:8\]
done
clear
View Answer play_arrow
question_answer 6) Direction of current induced in a wire moving in a magnetic field is found using
A)
Flemings left-hand rule
done
clear
B)
Flemings right hand rule
done
clear
C)
Amperes rule
done
clear
D)
Right hand clasp rule
done
clear
View Answer play_arrow
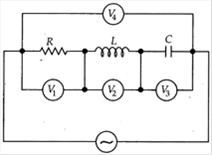
question_answer 7)
An ideal resistance R, ideal inductance L, ideal capacitance C and AC volt meters \[{{V}_{1}},{{V}_{2}},{{V}_{3}}\]and \[{{V}_{4}}\] are connected to an AC source as shown. At resonance,
A)
reading in \[{{V}_{3}}\] = reading in \[{{V}_{1}}\]
done
clear
B)
reading in \[{{V}_{1}}\] = reading in \[{{V}_{2}}\]
done
clear
C)
reading in \[{{V}_{2}}\] = reading in \[{{V}_{4}}\]
done
clear
D)
reading in \[{{V}_{2}}\] = reading in \[{{V}_{3}}\]
done
clear
View Answer play_arrow
question_answer 8) X-rays, gamma rays and microwaves travelling in vacuum have
A)
same wavelengths but different velocities
done
clear
B)
same frequency but different velocities
done
clear
C)
Same velocity but different wavelengths
done
clear
D)
Same velocity and same frequency
done
clear
View Answer play_arrow
question_answer 9) If n is the orbit number of the electron in a hydrogen atom, the correct statement among the following is
A)
Electron energy increases as n increases.
done
clear
B)
Hydrogen emits infrared rays for the electron transition from \[n=\infty \] to \[n=1\].
done
clear
C)
Electron energy is zero for \[n=1\].
done
clear
D)
Electron energy varies as \[{{n}^{2}}\].
done
clear
View Answer play_arrow
question_answer 10) In a Ruby laser, the colour of laser light is due to ............ atom.
A)
Oxygen
done
clear
B)
Aluminium
done
clear
C)
Xenon
done
clear
D)
chromium
done
clear
View Answer play_arrow
question_answer 11) The radius of \[_{29}C{{u}^{64}}\] nucleus in Fermi is (given \[{{R}_{0}}=1.2\times {{10}^{-15}}m\])
A)
\[4.8\]
done
clear
B)
\[1.2\]
done
clear
C)
\[7.7\]
done
clear
D)
\[9.6\]
done
clear
View Answer play_arrow
question_answer 12) In a radioactive decay, an element \[_{Z}{{X}^{A}}\] emits four \[\alpha \]-particles, three \[\beta \]-particles and eight gamma photons. The atomic number and mass number of the resulting final nucleus are
A)
\[Z-11,\,A-16\]
done
clear
B)
\[Z-5,\text{ }A-13\]
done
clear
C)
\[Z-5,\text{ }A-16\]
done
clear
D)
\[Z-8,\text{ }A-13\]
done
clear
View Answer play_arrow
question_answer 13) For a transistor, \[\beta =100\]. The value of a is
A)
\[1.01\]
done
clear
B)
\[0.99\]
done
clear
C)
\[100\]
done
clear
D)
\[0.01\]
done
clear
View Answer play_arrow
question_answer 14)
The following truth table with A and B as inputs is for ............ gate. A B Output 1 0 1 1 1 0 0 1 1 0 0 0
A)
AND
done
clear
B)
OR
done
clear
C)
XOR
done
clear
D)
NOR
done
clear
View Answer play_arrow
question_answer 15) n photons of wavelength \[\lambda \] are absorbed by a black body of mass m. The momentum gained by the body is
A)
\[\frac{h}{m\lambda }\]
done
clear
B)
\[\frac{mnh}{\lambda }\]
done
clear
C)
\[\frac{nh}{m\lambda }\]
done
clear
D)
\[\frac{nh}{\lambda }\]
done
clear
View Answer play_arrow
question_answer 16) A radioactive nucleus has specific binding energy\[{{E}_{1}}\]. It emits an \[\alpha \]-particle. The resulting nucleus has specific binding energy\[{{E}_{2}}\]. Then
A)
\[{{E}_{2}}={{E}_{1}}\]
done
clear
B)
\[{{E}_{2}}<{{E}_{1}}\]
done
clear
C)
\[{{E}_{2}}>{{E}_{1}}\]
done
clear
D)
\[{{E}_{2}}=0\]
done
clear
View Answer play_arrow
question_answer 17) The dimensional formula of physical quantity is \[{{M}^{a}}{{L}^{b}}{{T}^{c}}\] Then that physical quantity is
A)
surface tension if \[a=1,\,b=1,\,c=-2\]
done
clear
B)
Force if \[a=1,\,b=1,\,c=2\]
done
clear
C)
Angular frequency if \[a=0,\,b=0,\,c=-1\]
done
clear
D)
Spring constant if \[a=1,\,b=-1,\,c=-2\]
done
clear
View Answer play_arrow
question_answer 18) A person throws balls into air vertically upward in regular intervals of time of one second. The next ball is thrown when the velocity of the ball thrown earlier becomes zero. The height to which the balls rise is... (Assume, \[g=10m{{s}^{-2}}\])
A)
\[5m\]
done
clear
B)
\[10m\]
done
clear
C)
\[7.5m\]
done
clear
D)
\[20m\]
done
clear
View Answer play_arrow
question_answer 19) The circular motion of a particle with constant speed is
A)
periodic but not SHM
done
clear
B)
SHM but not periodic
done
clear
C)
periodic and also SHM
done
clear
D)
neither periodic nor SHM
done
clear
View Answer play_arrow
question_answer 20)
A planet moving around sun sweeps area \[{{A}_{1}}\] in 2 days, \[{{A}_{2}}\] in 3 days and \[{{A}_{3}}\]in 6 days. Then the relation between \[{{A}_{1}},{{A}_{2}}\] and \[{{A}_{3}}\] is
A)
\[3{{A}_{1}}=2{{A}_{2}}={{A}_{3}}\]
done
clear
B)
\[2{{A}_{1}}=3{{A}_{2}}=6{{A}_{3}}\]
done
clear
C)
\[3{{A}_{1}}=2{{A}_{2}}=6{{A}_{3}}\]
done
clear
D)
\[6{{A}_{1}}=3{{A}_{2}}=2{{A}_{3}}\]
done
clear
View Answer play_arrow
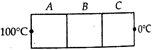
question_answer 21)
A, B and C are the three identical conductors but made from different materials. They are kept in contact as shown. Their thermal conductivities are K, 2K and \[\frac{K}{2}\]. The free end of A is at \[{{100}^{o}}C\] and the free end of C is at \[{{0}^{o}}C\]. During steady state, the temperature of the junction of A and B is nearly.....\[^{o}C\].
A)
\[71\]
done
clear
B)
\[29\]
done
clear
C)
\[63\]
done
clear
D)
\[37\]
done
clear
View Answer play_arrow
question_answer 22)
One mole of an ideal gas is taken from A to B from B to C and then back to A. The variation of its volume with temperature for that change is as shown. Its pressure at A is \[{{P}_{0}}\], volume is \[{{V}_{0}}\]. Then, the internal energy
A)
at A is more than at B
done
clear
B)
at C is less than at B
done
clear
C)
at B is more than at A
done
clear
D)
at A and B are equal
done
clear
View Answer play_arrow
question_answer 23) Which of the following is incorrect?
A)
If the wave is longitudinal, it must be a mechanical wave.
done
clear
B)
It the wave is mechanical, it may or may not be transverse wave.
done
clear
C)
Mechanical waves cannot propagate in vacuum.
done
clear
D)
Diffraction helps us to distinguish between sound wave and fight wave.
done
clear
View Answer play_arrow
question_answer 24) Intensity level of sound whose intensity is \[{{10}^{-8}}W{{m}^{-2}}\]is...dB
A)
\[8\]
done
clear
B)
\[4\]
done
clear
C)
\[40\]
done
clear
D)
\[80\]
done
clear
View Answer play_arrow
question_answer 25) A point source of light is kept below the surface of water \[\left( {{n}_{w}}=\frac{4}{3} \right)\] at a depth of \[\sqrt{7}m\]. The radius of the circular bright patch of light noticed on the surface of water is ... m.
A)
\[\frac{3}{\sqrt{7}}\]
done
clear
B)
\[3\]
done
clear
C)
\[\frac{\sqrt{7}}{3}\]
done
clear
D)
\[\sqrt{7}\]
done
clear
View Answer play_arrow
question_answer 26) A monochromatic beam of light is travelling from medium A of refractive index \[{{n}_{1}}\] to a medium B of refractive index \[{{n}_{2}}\]. In the medium A , there are x number of waves in certain distance. In the medium B, there are y number of waves in the same distance. Then, refractive index of medium A with respect to medium B is ...
A)
\[\frac{y}{x}\]
done
clear
B)
\[\sqrt{\frac{x}{y}}\]
done
clear
C)
\[\frac{x}{y-x}\]
done
clear
D)
\[\frac{x}{y}\]
done
clear
View Answer play_arrow
question_answer 27) In Youngs double slit experiment, fringes of width \[\beta \] are produced on a screen kept at a distance of 1 m from the slit. When the screen is moved away by\[5\times {{10}^{-2}}m\], fringe width changes by \[3\times {{10}^{-5}}m\]. The separation between the slits is\[1\times {{10}^{-3}}m\]. The wavelength of the light used is ... nm.
A)
\[500\]
done
clear
B)
\[600\]
done
clear
C)
\[700\]
done
clear
D)
\[400\]
done
clear
View Answer play_arrow
question_answer 28)
For sustained interference fringes in double slit experiment, essential condition/s is/are (1) sources must be coherent. (2) the intensities of the two sources must be equal.
Here, the correct option/s is/are
A)
both (1) and (2)
done
clear
B)
only (1)
done
clear
C)
only (2)
done
clear
D)
neither (1) nor (2)
done
clear
View Answer play_arrow
question_answer 29) In single slit experiment, the width of the slit is reduced. Then, the linear width of the principal Maxima ...
A)
increases but becomes less bright
done
clear
B)
decreases but becomes more bright
done
clear
C)
increases but becomes more bright
done
clear
D)
decreases but becomes less bright
done
clear
View Answer play_arrow
question_answer 30) In the uniform electric field of \[E=1\times {{10}^{4}}N{{C}^{-1}}\], an electron is accelerated from rest. The velocity of the electron when it has travelled a distance of \[2\times {{10}^{-2}}m\] is nearly ... \[m{{s}^{-1}}\] (\[\frac{e}{m}\] of electron \[=1.8\times {{10}^{11}}Ck{{g}^{-1}}\])
A)
\[1.6\times {{10}^{6}}\]
done
clear
B)
\[0.85\times {{10}^{6}}\]
done
clear
C)
\[0.425\times {{10}^{6}}\]
done
clear
D)
\[8.5\times {{10}^{6}}\]
done
clear
View Answer play_arrow
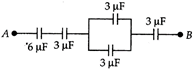
question_answer 31)
In this diagram, the P.D. between A and B is 60 V. The P.D. across \[6\mu F\] capacitor is ...V
A)
\[10\]
done
clear
B)
\[5\]
done
clear
C)
\[20\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
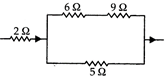
question_answer 32)
In this circuit, when certain current flows, the heat produced in \[5\Omega \], is \[4.05\text{ }J\]in a time t. The heat produced in \[2\Omega \]. coil in the same time interval is
A)
\[5.76\]
done
clear
B)
\[1.44\]
done
clear
C)
\[2.88\]
done
clear
D)
\[2.02\]
done
clear
View Answer play_arrow
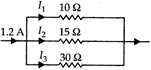
question_answer 33)
In this circuit, the value of \[{{I}_{2}}\] is
A)
\[0.2\text{ }A\]
done
clear
B)
\[0.3\text{ }A\]
done
clear
C)
\[0.4\text{ }A\]
done
clear
D)
\[0.6\text{ }A\]
done
clear
View Answer play_arrow
question_answer 34) A straight current carrying conductor is kept along the axis of circular loop carrying current. The force exerted by the straight conductor on the loop is .............
A)
perpendicular to the plane of the loop
done
clear
B)
in the plane of the loop, away from the center
done
clear
C)
in the plane of the loop, towards the center
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 35) A resistor of, an inductance of 0.5 H are in series with an a.c. which is given by \[V=100\sqrt{2}\sin \](10000. The power factor of the combination is
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\frac{1}{\sqrt{3}}\]
done
clear
C)
\[0.5\]
done
clear
D)
\[0.6\]
done
clear
View Answer play_arrow
question_answer 36) Pick out the wrong statement.
A)
The gain in the K.E. of the electron moving at right angles to the magnetic field is zero.
done
clear
B)
When an electron is shot at right angles to the electric field, it traces a parabolic path.
done
clear
C)
An electron moving in the direction of the electric field gains K.E.
done
clear
D)
An electron at rest experiences no force in the magnetic field.
done
clear
View Answer play_arrow
question_answer 37) A proton and an alpha particle are accelerated under the same potential difference. The ratio of de-Broglie wavelengths of the proton and the alpha particle is
A)
\[\sqrt{8}\]
done
clear
B)
\[\frac{1}{\sqrt{8}}\]
done
clear
C)
\[1\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 38) Spectrum of sunlight is an example for
A)
band emission spectrum
done
clear
B)
line absorption spectrum
done
clear
C)
continuous emission spectrum
done
clear
D)
continuous absorption spectrum
done
clear
View Answer play_arrow
question_answer 39) In hydrogen atom, electron excites from ground state to higher energy state and its orbital velocity is reduced to \[{{\left( \frac{1}{3} \right)}^{rd}}\] of its initial value. The radius of the orbit in the ground state is R. The radius of the orbit in that higher energy state is ...
A)
\[2R\]
done
clear
B)
\[3R\]
done
clear
C)
\[27R\]
done
clear
D)
\[9R\]
done
clear
View Answer play_arrow
question_answer 40) Decay constants of two radio-active samples A and B are 15x and 3x respectively. They have equal number of initial nuclei. The ratio of the number of nuclei left in A and B after a time \[\frac{1}{6x}\]is
A)
\[e\]
done
clear
B)
\[{{e}^{2}}\]
done
clear
C)
\[{{e}^{-1}}\]
done
clear
D)
\[{{e}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 41)
Mass numbers of the elements A, B, C and D are 30, 60, 90, and 120 respectively. The specific binding energy of them are 5 MeV, 8.5 MeV, 8 MeV and 7 MeV respectively. Then, in which of the following reaction/s energy is released? (1) \[D\to 2B\] (2) \[C\to B+A\] (3) \[B\to 2A\]
A)
only in (1)
done
clear
B)
in (2), (3)
done
clear
C)
in (1), (3)
done
clear
D)
in (1), (2) and (3)
done
clear
View Answer play_arrow
question_answer 42) Copper and Germanium are cooled from room temperature to 100 K. Then the resistance of
A)
Germanium decreases. Copper increases
done
clear
B)
Germanium decreases. Copper decreases
done
clear
C)
Germanium increases. Copper decreases
done
clear
D)
Germanium increases. Copper increases
done
clear
View Answer play_arrow
question_answer 43) The most stable particle in the Baryon group is
A)
neutron
done
clear
B)
proton
done
clear
C)
lamda particle
done
clear
D)
sigma particle
done
clear
View Answer play_arrow
question_answer 44) Frequencies of light incident on a system of scattering particles are in the ratio of \[1:2\]. Then, the intensity of scattered light in a particular direction is ...
A)
\[1:4\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:8\]
done
clear
D)
\[1:16\]
done
clear
View Answer play_arrow
question_answer 45) The ratio of the magnetic dipole moment to the angular momentum of the electron in the 1st orbit of hydrogen atom is
A)
\[\frac{e}{2m}\]
done
clear
B)
\[\frac{e}{m}\]
done
clear
C)
\[\frac{2m}{e}\]
done
clear
D)
\[\frac{m}{e}\]
done
clear
View Answer play_arrow
question_answer 46) Milk is an example for
A)
inelastic gel
done
clear
B)
foam
done
clear
C)
elastic gel
done
clear
D)
emulsion
done
clear
View Answer play_arrow
question_answer 47) A body of mass m is travelling with a velocity u. When a constant retarding force F is applied, it comes to rest after travelling a distance \[{{s}_{1}}\]. If the initial velocity is 2u, with the same force F, the distance travelled before it comes to rest is \[{{s}_{2}}\]. Then
A)
\[{{s}_{2}}=2{{s}_{1}}\]
done
clear
B)
\[{{s}_{2}}=\frac{{{s}_{1}}}{2}\]
done
clear
C)
\[{{s}_{2}}={{s}_{1}}\]
done
clear
D)
\[{{s}_{2}}=4{{s}_{1}}\]
done
clear
View Answer play_arrow
question_answer 48) A block kept on a rough surface starts sliding when the inclination of the surface is \[\theta \] with respect to the horizontal. The coefficient of static friction between the block and the surface is
A)
\[\sin \theta \]
done
clear
B)
\[\tan \theta \]
done
clear
C)
\[\cos \theta \]
done
clear
D)
\[sec\theta \]
done
clear
View Answer play_arrow
question_answer 49) Two bodies of masses m, and m^ are acted upon by a constant force F for a time t. They start from rest and acquire kinetic energies \[{{E}_{1}}\] and \[{{E}_{2}}\] respectively. Then \[\frac{{{E}_{1}}}{{{E}_{2}}}\] is
A)
\[\frac{{{m}_{1}}}{{{m}_{2}}}\]
done
clear
B)
\[\frac{{{m}_{2}}}{{{m}_{1}}}\]
done
clear
C)
\[1\]
done
clear
D)
\[\frac{\sqrt{{{m}_{1}}{{m}_{2}}}}{{{m}_{1}}+{{m}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 50) The X and Y components of a force F acting at \[{{30}^{o}}\] to x-axis are respectively
A)
\[\frac{F}{\sqrt{2}},F\]
done
clear
B)
\[\frac{F}{2},\frac{\sqrt{3}}{2}F\]
done
clear
C)
\[\frac{\sqrt{3}}{2}F,\frac{1}{2}F\]
done
clear
D)
\[F,\frac{F}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 51) Spheres of iron and lead having same mass are completely immersed in water. Density of lead is more than that of iron. Apparent loss of weight is \[{{W}_{1}}\]for iron sphere and \[{{W}_{2}}\]for lead sphere. Then \[\frac{{{W}_{1}}}{{{W}_{2}}}\] is
A)
\[=1\]
done
clear
B)
between 0 and 1
done
clear
C)
\[=0\]
done
clear
D)
\[>1\]
done
clear
View Answer play_arrow
question_answer 52) A hot body is allowed to cool. The surrounding temperature is constant at\[{{30}^{o}}C\]. The body takes time t, to cool from \[{{90}^{o}}C\] to \[{{89}^{o}}C\] and time \[{{t}_{2}}\] to cool from \[{{60}^{o}}C\] to \[{{59.5}^{o}}C\]. Then,
A)
\[{{t}_{2}}=2{{t}_{1}}\]
done
clear
B)
\[{{t}_{2}}=\frac{{{t}_{1}}}{2}\]
done
clear
C)
\[{{t}_{2}}=4{{t}_{1}}\]
done
clear
D)
\[{{t}_{2}}={{t}_{1}}\]
done
clear
View Answer play_arrow
question_answer 53) A particle executes SHM with amplitude \[0.2m\]and time period 24 s. The time required for it to move from the mean position to a point 0.1 m from the mean position is
A)
\[2s\]
done
clear
B)
\[3s\]
done
clear
C)
\[8s\]
done
clear
D)
\[12s\]
done
clear
View Answer play_arrow
question_answer 54) White light is incident normally on a glass slab. Inside the glass slab,
A)
red light travels faster than other colours.
done
clear
B)
violet light travels faster than other colours.
done
clear
C)
yellow light travels faster than other colours.
done
clear
D)
all colours travel with the same speed.
done
clear
View Answer play_arrow
question_answer 55)
Two thin plano-convex lenses each of focal length f are placed as shown in the figure. The ratio of their effective focal lengths in the three cases is
A)
\[1:2:3\]
done
clear
B)
\[1:2:1\]
done
clear
C)
\[1:1:1\]
done
clear
D)
\[3:2:1\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 56) If the two slits in Youngs double slit experiment are of unequal width, then
A)
the bright fringes will have unequal spacing.
done
clear
B)
the bright fringes will have unequal brightness.
done
clear
C)
the fringes do not appear.
done
clear
D)
the dark fringes are not perfectly dark.
done
clear
View Answer play_arrow
question_answer 57) The phenomenon of polarization shows that light has ............ nature.
A)
particle
done
clear
B)
transverse
done
clear
C)
longitudinal
done
clear
D)
dual
done
clear
View Answer play_arrow
question_answer 58) Acceleration of a charged particle of charge q and mass m moving in a uniform electric field of strength E is
A)
\[\frac{qE}{m}\]
done
clear
B)
\[\frac{m}{qE}\]
done
clear
C)
\[mqE\]
done
clear
D)
\[\frac{q}{mE}\]
done
clear
View Answer play_arrow
question_answer 59) Two fixed charges A and B of \[5\mu C\] each are separated by a distance of 6 m. C is the mid point of the line joining A and B. A charge Q of \[-5\mu C\] is shot perpendicular to the line joining A and B through C with a kinetic energy of 0.06 J. The charge Q comes to rest at a point D. The distance CD is
A)
\[3m\]
done
clear
B)
\[\sqrt{3}m\]
done
clear
C)
\[3\sqrt{3}m\]
done
clear
D)
\[4m\]
done
clear
View Answer play_arrow
question_answer 60) A capacitor of capacitance of \[10\mu F\] is charged to 10 V. The energy stored in it is
A)
\[100\mu J\]
done
clear
B)
\[500\mu J\]
done
clear
C)
\[1000\mu J\]
done
clear
D)
\[1\mu J\]
done
clear
View Answer play_arrow
question_answer 61) A first order reaction is 60% complete in 20 minutes. How long will the reaction take to be 84% complete?
A)
\[54mins\]
done
clear
B)
\[68mins\]
done
clear
C)
\[40mins\]
done
clear
D)
\[76mins\]
done
clear
View Answer play_arrow
question_answer 62) A given sample of milk turns sour at room temperature \[({{27}^{o}}C)\] in 5 hours. In a refrigerator at \[-{{3}^{o}}C\], it can be stored 10 times longer. The energy of activation for the souring of milk is
A)
\[2.303\times 10\text{ }R\text{ }kJ.mo{{l}^{-1}}\]
done
clear
B)
\[2.303\times 5\text{ }R\text{ }kJ.mo{{l}^{-1}}\]
done
clear
C)
\[2.303\times 3\text{ }R\text{ }kJ.mo{{l}^{-1}}\]
done
clear
D)
\[2.303\times 2.7\text{ }R\text{ }kJ.mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 63) At 300 K, a gaseous reaction: \[A\xrightarrow{{}}B+C\]was found to follow first order kinetics. Starting with pure A, the total pressure at the end of 20 minutes was 100 mm of Hg. The total pressure after the completion of the reaction is 180 mm of Hg. The partial pressure of A (in mm of Hg) is
A)
\[100\]
done
clear
B)
\[90\]
done
clear
C)
\[180\]
done
clear
D)
\[80\]
done
clear
View Answer play_arrow
question_answer 64) From the Ellingham graphs on carbon, which of the following statements is false?
A)
\[C{{O}_{2}}\] is more stable than \[CO\] at less than 983 K
done
clear
B)
\[CO\] reduces \[F{{e}_{2}}{{O}_{3}}\] to Fe at less than 983 K
done
clear
C)
\[CO\] is less stable than \[C{{O}_{2}}\] at more than 983 K
done
clear
D)
\[CO\] reduces \[F{{e}_{2}}{{O}_{3}}\] to Fe in the reduction zone of Blast furnace
done
clear
View Answer play_arrow
question_answer 65) Which of the following is a negatively charged bidentate ligand?
A)
Dimethyl glyoximato
done
clear
B)
Cyano
done
clear
C)
Ethylene diamine
done
clear
D)
Acetato
done
clear
View Answer play_arrow
question_answer 66) The secondary valency of platinum in tetra mine dichloroplatinum (IV) chloride is
A)
\[+4\]
done
clear
B)
\[+2\]
done
clear
C)
\[3\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 67) Which one of the following has a magnetic moment of\[1.75B.M\]?
A)
\[T{{i}^{3+}}\]
done
clear
B)
\[{{V}^{3+}}\]
done
clear
C)
\[C{{r}^{3+}}\]
done
clear
D)
\[F{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 68) The correct order of ionisation energy of C, N,O and F is
A)
\[F<N<C<O\]
done
clear
B)
\[C<N<O<F\]
done
clear
C)
\[C<O<N<F\]
done
clear
D)
\[F<O<N<C\]
done
clear
View Answer play_arrow
question_answer 69) The correct set of four quantum numbers for the outermost electron of sodium \[(Z=11)\] is
A)
\[3,1,0,\frac{1}{2}\]
done
clear
B)
\[3,1,1,\frac{1}{2}\]
done
clear
C)
\[3,2,1,\frac{1}{2}\]
done
clear
D)
\[3,0,0,\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 70) The ore that is concentrated by the Froth Floatation process is
A)
Chalcopyrites
done
clear
B)
Cryolite
done
clear
C)
Cuprite
done
clear
D)
Calamine
done
clear
View Answer play_arrow
question_answer 71) The equivalent mass of a certain bivalent metal is 20. The molecular mass of its anhydrous chloride is
A)
\[91\]
done
clear
B)
\[111\]
done
clear
C)
\[55.5\]
done
clear
D)
\[75.5\]
done
clear
View Answer play_arrow
question_answer 72) 2 moles of \[{{N}_{2}}{{O}_{4(g)}}\] is kept in a closed container at 298 K and under 1 atm pressure. It is heated to 596 K when 20% by mass of \[{{N}_{2}}{{O}_{4(g)}}\]) decomposes to \[N{{O}_{2}}\]. The resulting pressure is
A)
\[2.4atm\]
done
clear
B)
\[1.2atm\]
done
clear
C)
\[4.8atm\]
done
clear
D)
\[2.8atm\]
done
clear
View Answer play_arrow
question_answer 73) Sucrose is not a reducing sugar since
A)
it is chemically stable
done
clear
B)
it contains no free aldehyde or keto group adjacent to a
group
done
clear
C)
it is built up of a fructose unit
done
clear
D)
it is optically active
done
clear
View Answer play_arrow
question_answer 74) Which one of the following contains ionic, covalent and co-ordinate bonds?
A)
\[NaOH\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[NaCN\]
done
clear
D)
\[NaNC\]
done
clear
View Answer play_arrow
question_answer 75) Dialysis can be used to separate
A)
glucose and fructose
done
clear
B)
protein and starch
done
clear
C)
glucose and protein
done
clear
D)
glucose and \[NaCl\]
done
clear
View Answer play_arrow
question_answer 76) The percentage of p-character of the hybrid orbital in graphite and diamond are respectively:
A)
\[33\] and \[25\]
done
clear
B)
\[50\] and \[75\]
done
clear
C)
67 and \[75\]
done
clear
D)
\[33\] and \[75\]
done
clear
View Answer play_arrow
question_answer 77) A gas expands from a volume of \[1{{m}^{3}}\]to a volume of \[2{{m}^{3}}\]against an external pressure of \[{{10}^{5}}\text{ }N\text{ }{{m}^{-2}}\] The work done by the gas will be
A)
\[{{10}^{5}}kJ\]
done
clear
B)
\[{{10}^{2}}kJ\]
done
clear
C)
\[{{10}^{2}}J\]
done
clear
D)
\[{{10}^{3}}J\]
done
clear
View Answer play_arrow
question_answer 78) The mass of a non-volatile solute of molar man \[40g\text{ }mo{{l}^{-1}}\]that should be dissolved in 114 g octane to lower its vapour pressure by 20% is
A)
\[10g\]
done
clear
B)
\[11.4g\]
done
clear
C)
\[9.8g\]
done
clear
D)
\[12.8g\]
done
clear
View Answer play_arrow
question_answer 79) During the adsorption of a gas on the surface a solid, which of the following is true?
A)
\[\Delta G<0,\text{ }\Delta H>0,\text{ }\Delta S<0\]
done
clear
B)
\[\Delta G>0,\text{ }\Delta H<0,\text{ }\Delta S<0\]
done
clear
C)
\[\Delta G<0,\Delta H<0,\Delta S<0\]
done
clear
D)
\[\Delta G<0,\Delta H<0,\Delta S>0\]
done
clear
View Answer play_arrow
question_answer 80) The approximate time duration in hours to electroplate 30 g of calcium from molten calcium chloride using a current of 5 amp is [At. mass of Ca = 40]
A)
\[8\]
done
clear
B)
\[80\]
done
clear
C)
\[10\]
done
clear
D)
\[16\]
done
clear
View Answer play_arrow
question_answer 81) The pH of the solution obtained by mixing 100 mL of a solution of \[pH=3\]with 400 mL of solution of \[pH=4\]is
A)
\[3-log\text{ }2.8\]
done
clear
B)
\[7\text{ }-log\text{ }2.8g\]
done
clear
C)
\[4-log\text{ }2.8\]
done
clear
D)
\[5-log\text{ }2.8\]
done
clear
View Answer play_arrow
question_answer 82) The equilibrium constant of the reaction : \[{{A}_{(s)}}+2B_{(aq)}^{+}\rightleftharpoons A_{(aq)}^{2+}+2{{B}_{(s)}};E_{cell}^{o}=0.0295V\]is \[\left[ \frac{2.303RT}{F}=0.059 \right]\]
A)
\[10\]
done
clear
B)
\[2\times {{10}^{2}}\]
done
clear
C)
\[3\times {{10}^{2}}\]
done
clear
D)
\[2\times {{10}^{5}}\]
done
clear
View Answer play_arrow
question_answer 83) An oxygen containing organic compound was found to contain 52% carbon and 13% of hydrogen. Its vapour density is 23. The compound reacts with sodium metal to liberate hydrogen. A functional isomer of this compound is
A)
Ethanol
done
clear
B)
Ethanal
done
clear
C)
Methoxy methane
done
clear
D)
Methoxy ethane
done
clear
View Answer play_arrow
question_answer 84) Which one of the following is not true regarding electromeric effect?
A)
It results in the appearance of partial charges on the carbon atoms.
done
clear
B)
It is a temporary effect.
done
clear
C)
It operates on multiple bonds.
done
clear
D)
It requires an attacking reagent.
done
clear
View Answer play_arrow
question_answer 85) Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence of dry ether?
A)
Ethane
done
clear
B)
Diphenyl
done
clear
C)
Propane
done
clear
D)
Toluene
done
clear
View Answer play_arrow
question_answer 86) Power alcohol is a mixture of
A)
80% Petrol \[+\] 20% Benzene \[+\] Small quantity of Ethanol
done
clear
B)
80% Petrol \[+\] 20% Ethanol \[+\] Small quantity of Benzene
done
clear
C)
80% Ethanol \[+\] 20% Benzene \[+\]Small quantity of Petrol
done
clear
D)
50% Petrol \[+\] 50% Ethanol \[+\] Small quantity of Benzene
done
clear
View Answer play_arrow
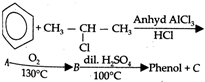
question_answer 87)
Identity C in the following
A)
Water
done
clear
B)
Ethanol
done
clear
C)
Propanone
done
clear
D)
Cumene Hydro peroxide
done
clear
View Answer play_arrow
question_answer 88) \[20mL\]of methane is completely burnt using \[50mL\]of oxygen. The volume of the gas left after cooling to room temperature is
A)
\[80mL\]
done
clear
B)
\[40mL\]
done
clear
C)
\[60mL\]
done
clear
D)
\[30mL\]
done
clear
View Answer play_arrow
question_answer 89) \[100mL\]of \[0.1M\]acetic add is completely neutralized using a standard solution of \[NaOH\]. The volume of ethane obtained at STP after the complete electrolysis of the resulting solution is
A)
\[112mL\]
done
clear
B)
\[56mL\]
done
clear
C)
\[224mL\]
done
clear
D)
\[560mL\]
done
clear
View Answer play_arrow
question_answer 90) Saccharin, an artificial sweetener, is manufactured from
A)
Cellulose
done
clear
B)
Toluene
done
clear
C)
Cyclohexane
done
clear
D)
Starch
done
clear
View Answer play_arrow
question_answer 91) Which of the following is not true for \[{{S}_{N}}1\] reaction?
A)
Favoured by polar solvents
done
clear
B)
\[{{3}^{o}}\]-alkyl halides generally react through \[{{S}_{N}}1\] reaction.
done
clear
C)
The rate of the reaction does not depend upo the molar concentration of the nucleophile.
done
clear
D)
\[{{1}^{o}}\]-alkyl halides generally react through \[{{S}_{N}}1\]reaction.
done
clear
View Answer play_arrow
question_answer 92) Oil of winter green is
A)
An ester
done
clear
B)
A carboxylic acid
done
clear
C)
An alcohol
done
clear
D)
A ketone
done
clear
View Answer play_arrow
question_answer 93) An organic compound A burns with a sooty flame. It is negative towards Tollens reagent test and positive for Borsches reagent test. The compound A is
A)
Benz aldehyde
done
clear
B)
Acetophenone
done
clear
C)
Acetone
done
clear
D)
Salicylic acid
done
clear
View Answer play_arrow
question_answer 94)
For a reaction: \[A+B\xrightarrow{{}}\]Products, the rate of the reaction at various concentrations are given below: Expt [A] [B] \[~rate(mol\text{ }d{{m}^{-3}}\text{ }{{s}^{-1}})\] 1 0.2 0.2 2 2 0.2 0.4 4 3 0.6 04 36
The rate law for the above reaction is ,
A)
\[r=k{{[A]}^{2}}[B]\]
done
clear
B)
\[r=k[A]{{[B]}^{2}}\]
done
clear
C)
\[r=k{{[A]}^{3}}[B]\]
done
clear
D)
\[r=k{{[A]}^{2}}{{[B]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 95) Which one of the following has no unpaired electrons?
A)
\[{{O}_{2}}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[O_{2}^{+}\]
done
clear
D)
\[O_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 96) The atomic number of cobalt is 27. The EAN of cobalt in \[N{{a}_{3}}[Co{{(N{{O}_{2}})}_{4}}C{{l}_{2}}]\] is
A)
\[35\]
done
clear
B)
\[24\]
done
clear
C)
\[36\]
done
clear
D)
\[34\]
done
clear
View Answer play_arrow
question_answer 97) The spin only magnetic moment of \[N{{i}^{2+}}\] in aqueous solution would be [At No. of \[Ni=28\]]
A)
\[\sqrt{6}B.M\]
done
clear
B)
\[\sqrt{15}B.M\]
done
clear
C)
\[\sqrt{2}B.M\]
done
clear
D)
\[\sqrt{8}B.M\]
done
clear
View Answer play_arrow
question_answer 98) Impossible orbital among the following is
A)
\[2s\]
done
clear
B)
\[3f\]
done
clear
C)
\[2p\]
done
clear
D)
\[4d\]
done
clear
View Answer play_arrow
question_answer 99) The total number of electrons in 18 mL of water (density\[=1g\text{ }m{{L}^{-1}}\]) is
A)
\[6.02\times {{10}^{23}}\]
done
clear
B)
\[6.02\times {{10}^{25}}\]
done
clear
C)
\[6.02\times {{10}^{24}}\]
done
clear
D)
\[6.02\times 18\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 100) The number of moles of hydrogen that can be added to 1 mole of an oil is the highest in
A)
Linseed oil
done
clear
B)
Groundnut oil
done
clear
C)
Sunflower seed oil
done
clear
D)
Mustard oil
done
clear
View Answer play_arrow
question_answer 101) The reaction between sodium and water can be made less vigorous by
A)
Lowering the temperature
done
clear
B)
Adding a little alcohol
done
clear
C)
Amalgamating sodium
done
clear
D)
Adding a little acetic acid
done
clear
View Answer play_arrow
question_answer 102) All colloidal dispersions have
A)
very high osmotic pressure
done
clear
B)
Low osmotic pressure
done
clear
C)
No osmotic pressure
done
clear
D)
High osmotic pressure
done
clear
View Answer play_arrow
question_answer 103) Silver iodide is used for producing artificial rain because \[AgI\]
A)
is easy to spray at high altitude
done
clear
B)
is easy to synthesize
done
clear
C)
has crystal structure similar to ice
done
clear
D)
is insoluble in water
done
clear
View Answer play_arrow
question_answer 104) The equilibrium constant of a reaction is \[0.008\] at 298 K. The standard free energy change of the reaction at the same temperature is
A)
\[+11.96\text{ }kJ\]
done
clear
B)
\[-11.96\text{ }kJ\]
done
clear
C)
\[-5.43\text{ }kJ\]
done
clear
D)
\[-8.46\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 105) The function of potassium ethyl xanthate in froth floatation process is to make the ore
A)
attracted towards water
done
clear
B)
water repellant
done
clear
C)
lighter
done
clear
D)
heavier
done
clear
View Answer play_arrow
question_answer 106) The correct order of electronegativities of N, O, F and P is
A)
\[F>N>P>O\]
done
clear
B)
\[F>O>P>N\]
done
clear
C)
\[F>O>N>P\]
done
clear
D)
\[N>O>F>P\]
done
clear
View Answer play_arrow
question_answer 107) The s-block element used as a catalyst in the manufacture of Buna-S rubber is
A)
\[Mg\]
done
clear
B)
\[Ca\]
done
clear
C)
\[Ba\]
done
clear
D)
\[Na\]
done
clear
View Answer play_arrow
question_answer 108) Which of the following is not a characteristic of a covalent compound?
A)
Low melting point
done
clear
B)
No definite geometry
done
clear
C)
Insoluble in polar solvent
done
clear
D)
Small difference in electronegativity between the combining atoms.
done
clear
View Answer play_arrow
question_answer 109) The volume of \[0.1M\]oxalic acid that can be completely oxidized by \[20mL\]of \[0.025M\,\,KMn{{O}_{4}}\] solution is
A)
\[125mL\]
done
clear
B)
\[25mL\]
done
clear
C)
\[12.5mL\]
done
clear
D)
\[37.5mL\]
done
clear
View Answer play_arrow
question_answer 110) A ligand is
A)
Lewis acid
done
clear
B)
Bronsted acid
done
clear
C)
either a Lewis acid or a Lewis base
done
clear
D)
Lewis base
done
clear
View Answer play_arrow
question_answer 111) The vapour pressures of two liquids A and B in their pure states are in ratio of\[1:2\]. A binary solution of A and 5 contains A and B in the mole proportion of \[1:2\]. The mole fraction of A in the vapour phase of the solution will be
A)
\[0.33\]
done
clear
B)
\[0.2\]
done
clear
C)
\[0.25\]
done
clear
D)
\[0.52\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following statements is true?
A)
The total entropy of the universe remains constant.
done
clear
B)
The total entropy of the universe is continuously decreasing.
done
clear
C)
The total energy of the universe is continuously decreasing.
done
clear
D)
The total energy of the universe remains constant.
done
clear
View Answer play_arrow
question_answer 113) \[5mL\]of \[0.4N\text{ }NaOH\]is mixed with \[20mL\]of\[0.1N\text{ }HCl\]. The pH of the resulting solution will be
A)
\[6\]
done
clear
B)
\[7\]
done
clear
C)
\[8\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 114) On adding which of the following, the pH of \[20mL\] of \[0.1N\text{ }HCl\]will not alter?
A)
\[1mL\]of \[1N\text{ }HCl\]
done
clear
B)
\[20mL\]of distilled water
done
clear
C)
\[1mL\]of \[0.1N\text{ }NaOH\]
done
clear
D)
\[500mL\]of \[HCl\]of \[pH=1\]
done
clear
View Answer play_arrow
question_answer 115) Which one of the following has a potential more than zero?
A)
\[Pt,\frac{1}{2}{{H}_{2}}(1atm)|HCl(1M)\]
done
clear
B)
\[Pt,\frac{1}{2}{{H}_{2}}(1atm)|HCl(2M)\]
done
clear
C)
\[Pt,\frac{1}{2}{{H}_{2}}(1atm)|HCl(0.1M)\]
done
clear
D)
\[Pt,\frac{1}{2}{{H}_{2}}(1atm)|HCl(0.5M)\]
done
clear
View Answer play_arrow
question_answer 116) HCHO was treated with a reagent X. The product formed upon hydrolysis in the presence of an acid gave\[{{C}_{2}}{{H}_{5}}OH\]. The reagent X is
A)
Aqueous \[KOH\]
done
clear
B)
Alcoholic \[KOH\]
done
clear
C)
Alcoholic \[KCN\]
done
clear
D)
\[C{{H}_{3}}MgI\]
done
clear
View Answer play_arrow
question_answer 117) Benzylamine is a stronger base than aniline because
A)
The lone pair of electrons on the nitrogen atom in benzylamine is delocalised.
done
clear
B)
The lone pair of electrons on the nitrogen atom in aniline is delocalised.
done
clear
C)
The lone pair of electrons on the nitrogen atom in aniline is not involved in resonance.
done
clear
D)
Benzylamine has a higher molecular mass than aniline.
done
clear
View Answer play_arrow
question_answer 118) The relative acidic strangths of benzoic acid, o-toluic acid and p-toluic acid is of the decreasing order :
A)
p-toluic acid > o-toluic acid > benzoic acid
done
clear
B)
o-toluic acid > p-toluic acid > benzoic acid
done
clear
C)
p-toluic acid > benzoic acid > o-toluic acid
done
clear
D)
o-toluic acid > benzoid acid > p-toluic acid
done
clear
View Answer play_arrow
question_answer 119) The \[C-H\] bond and \[C-C\] bond in ethane are formed by which of the following types of overlap?
A)
\[s{{p}^{3}}-s\]and \[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}-s\]and \[s{{p}^{2}}-s{{p}^{2}}\]
done
clear
C)
\[sp-s\]and \[sp-sp\]
done
clear
D)
\[p-s\]and \[p-p\]
done
clear
View Answer play_arrow
question_answer 120)
The IUPAC name of
A)
4-Hydroxy-2-pentanone
done
clear
B)
2-Hydroxy-4-pentanone
done
clear
C)
2-0xo-4-pentanol
done
clear
D)
4-Keto-2-pentanol
done
clear
View Answer play_arrow
question_answer 121) Secondary cortex is also known as
A)
phellem
done
clear
B)
phelloderm
done
clear
C)
phellogen
done
clear
D)
bark.
done
clear
View Answer play_arrow
question_answer 122) Pteridophytes are called vascular cryptogams, because they are non-seeded plants containing
A)
xylem and phloem
done
clear
B)
only xylem
done
clear
C)
only phloem
done
clear
D)
neither xylem nor phloem.
done
clear
View Answer play_arrow
question_answer 123) The enzymes which are absolutely necessary for recombinant DNA technology are
A)
restriction endonucleases and topoisomerases
done
clear
B)
endonucleases and polymerases
done
clear
C)
restriction endonucleases and ligases
done
clear
D)
peptidases and ligases.
done
clear
View Answer play_arrow
question_answer 124) Stomata on the surface of the leaf, open by
A)
decreasing the solute concentration in the guard cells
done
clear
B)
increasing the solute concentration in the guard cells
done
clear
C)
weakening of the cell walls of the guard cells to allow them to stretch
done
clear
D)
increasing the water potential in the guard cells.
done
clear
View Answer play_arrow
question_answer 125) Read the two statements A and B and identify the correct option from those given below. Statement A: Agrobacterium tumefaciens is the causative agent of crown gall disease of dicots. Statement B: Agrobacterium tumefaciens causes infection by entering the plant through wounds and injuries.
A)
Statement A is correct and B is wrong.
done
clear
B)
Statement B is correct and A is wrong.
done
clear
C)
Both statements A and B are correct.
done
clear
D)
Both statements A and B are wrong.
done
clear
View Answer play_arrow
question_answer 126) Which of the following is the correct pathway of absorbed water in the roots of plants?
A)
Soil water \[\to \] root hair cell \[\to \] cortical cells pericycle \[\to \] passage cells \[\to \] xylem.
done
clear
B)
Soil water \[\to \] root hair cell \[\to \] passage cells cortical cells\[\to \] xylem \[\to \] pericycle.
done
clear
C)
Soil water \[\to \] root hair cell \[\to \] pericycle \[\to \]cortical cells \[\to \] passage cells \[\to \] xylem.
done
clear
D)
Soil water \[\to \] root hair cell \[\to \] cortical cells passage cells \[\to \] pericycle \[\to \] xylem.
done
clear
View Answer play_arrow
question_answer 127) Usually, the whorl in a flower that attracts insects and protects the essential parts is
A)
calyx
done
clear
B)
androecium
done
clear
C)
gynoecium
done
clear
D)
corolla.
done
clear
View Answer play_arrow
question_answer 128) Vein loading is the active transport of sugars from
A)
mesophyll cells to vessels
done
clear
B)
vessels to mesophyll cells
done
clear
C)
mesophyll cells to sieve tubes
done
clear
D)
sieve tubes to mesophyll cells.
done
clear
View Answer play_arrow
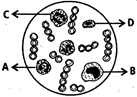
question_answer 129)
Study the diagram given below and identify the cells labelled as A, B, C and D, and choose the correct option.
A)
A = Eosinophil, B = Erythrocyte, C= Neutrophil and D = Basophil
done
clear
B)
A = Eosinophil, B = Lymphocyte, C = Neutrophil and D = Monocyte
done
clear
C)
A=Erythrocyte, B=Basophil, C=Neutrophil and D = Lymphocyte
done
clear
D)
A = Eosinophil, B = Monocyte, C = Neutrophil and D = Lymphocyte
done
clear
View Answer play_arrow
question_answer 130) The sexually transmitted disease, that can affect both the male and the female genitals and may damage the eyes of babies born of infected mothers is
A)
AIDS
done
clear
B)
syphilis
done
clear
C)
gonorrhoea
done
clear
D)
hepatitis.
done
clear
View Answer play_arrow
question_answer 131) Chemiosmotic theory of ATP synthesis in the mitochondrion is based on
A)
\[C{{a}^{++}}\] gradient
done
clear
B)
\[{{K}^{+}}\] gradient
done
clear
C)
\[{{H}^{+}}\] gradient
done
clear
D)
\[N{{a}^{+}}\] gradient.
done
clear
View Answer play_arrow
question_answer 132) Following are few characters of a disorder in human body. [A] Inflammation of the mucous membrane of nasal passage. [B] Watery secretions by mucous glands. [C] Continuous sneezing. [D] Eye watering. [E] Rise in body temperature. Identify the disorder from the choices given below.
A)
Bronchial asthma
done
clear
B)
Rhinitis
done
clear
C)
Bronchial carcinoma
done
clear
D)
Emphysema.
done
clear
View Answer play_arrow
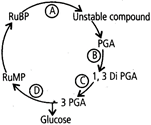
question_answer 133)
In a condensed schematic representation of dark reaction of photosynthesis given below, steps are indicated by alphabets. Select the option where the alphabets are correctly identified.
A)
A = \[C{{O}_{2}}\] fixation, B = Reduction, C = Phosphorylation, D = Regeneration
done
clear
B)
A = Regeneration, B = \[C{{O}_{2}}\] fixation, C = Reduction, D = Phosphorylation
done
clear
C)
A = \[C{{O}_{2}}\] fixation, B = Phosphorylation, C = Reduction, D = Regeneration
done
clear
D)
A = \[C{{O}_{2}}\] fixation, B = Phosphorylation, C = Regeneration, D = Reduction.
done
clear
View Answer play_arrow
question_answer 134)
Match the plants of economic importance given under column I with their scientific names given under column II and choose the correct option from the codes given below. Column I Column II A. Spices p. Syzygium aromaticum B. Pulses q. Cajanus cajan C. Medicinal r. Adhatoda vasica D. Cereals s. Sorghum vulgare t.Thea chinensis
A)
\[A=p,\text{ }B=r,\text{ }C=s,\text{ }D=t\]
done
clear
B)
\[A=p,B=s,C=r,D=q\]
done
clear
C)
\[A=t,B=r,C=q,D=p\]
done
clear
D)
\[A=p,\text{ }B=q,\text{ }C=r,\text{ }D=s\]
done
clear
View Answer play_arrow
question_answer 135) If father shows normal genotype and mother shows a carrier trait for haemophilia then
A)
all the female off springs will be normal
done
clear
B)
all the female, off springs will be carriers
done
clear
C)
a male offspring has 50% chance of active disease
done
clear
D)
a female offspring has probability of 50% to have active disease.
done
clear
View Answer play_arrow
question_answer 136) According to Best and Taylorss theory, which of the following does not play any role in blood clotting?
A)
Prothrombin
done
clear
B)
Fibrinogen
done
clear
C)
Platelets
done
clear
D)
Calcium ions.
done
clear
View Answer play_arrow
question_answer 137) Which of the following is not a character of cancerous tissues in our body?
A)
Contact inhibition
done
clear
B)
Neoplasia
done
clear
C)
Metastasis
done
clear
D)
Inability for differentiation -
done
clear
View Answer play_arrow
question_answer 138) Which of the following statements is not true for Nostoc?
A)
It is prokaryotic.
done
clear
B)
It is autotrophic.
done
clear
C)
It is filamentous,
done
clear
D)
It is macroscopic.
done
clear
View Answer play_arrow
question_answer 139) The system of classification of-plants proposed by which of these two botanists is claimed to be a natural system?
A)
Engler and Prantl
done
clear
B)
Bentham and Hooker
done
clear
C)
Aristotle and Theophrastus
done
clear
D)
Darwin and Wallace
done
clear
View Answer play_arrow
question_answer 140)
Match the entries in Column I with those of Column II and choose the correct answer. Column I Column II A. Cleistogamy m. Insect pollination B. Geitonogamy n. Bud pollination C. Entomophily o. Pollination between flowers in the same plant D. Xenogamy p. Wind pollination q. Cross pollination
A)
\[A-o;B-m;C-q;D-n\]
done
clear
B)
\[A-m;B-q;C-n;D-o\]
done
clear
C)
\[A-n;B-o;C-m;D-q\]
done
clear
D)
\[A-q;B-p;C-o;D-n\]
done
clear
View Answer play_arrow
question_answer 141) The host for Cercospora personata belongs to this family of angiosperms.
A)
Gramineae
done
clear
B)
Leguminosae
done
clear
C)
Malvaceae
done
clear
D)
Asclepiadaceae
done
clear
View Answer play_arrow
question_answer 142) The final stage in the tissue culture programme before the new plants are taken out for cultivation: in the fields is known as
A)
micro propagation
done
clear
B)
hardening
done
clear
C)
caulogenesis
done
clear
D)
embryogenesis
done
clear
View Answer play_arrow
question_answer 143) An osmometer is filled with 0.5 M solution \[NaCl\] in water. In which of the following to Buttons it must be immersed in order to make it shrink?
A)
\[0.5M\]solution
done
clear
B)
\[0.05M\]solution
done
clear
C)
Distilled water
done
clear
D)
\[0.75M\]solution
done
clear
View Answer play_arrow
question_answer 144) Perishable vegetables can be maintained fresh for a longer period by spraying them with a solution of
A)
ABA -
done
clear
B)
cytokinin
done
clear
C)
ethephon
done
clear
D)
phenyl mercuric acetate
done
clear
View Answer play_arrow
question_answer 145) The prebiotic atmosphere of the earth was of a reducing nature. It was transformed into an oxidizing atmosphere of present day due to the emergence of
A)
cyanobacteria
done
clear
B)
angiosperms
done
clear
C)
photosyntnetic bacteria
done
clear
D)
eukaryotic algae
done
clear
View Answer play_arrow
question_answer 146)
Match the contraceptive methods given under Column I with their examples given under Column II. Select the correct choice from those given below. Column I Column II A. Chemical p. Tubectomy and Vasectomy B. lUDs q. Copper T and Loop C. Barriers r. Condom and Cervical cap D. Sterilization s. Spermicidal jelly and foam t. Coitus interruptus and calendar method
A)
\[A=s,B=q,C=r,D=p\]
done
clear
B)
\[A=s,B=t,C=q,D=r\]
done
clear
C)
\[A=p,\text{ }B=r,C=q,D=t\]
done
clear
D)
\[A=s,B=q,C=t,D=p\]
done
clear
View Answer play_arrow
question_answer 147) One of the following movements in our body is not completely involuntary. Identify it.
A)
Deglutition
done
clear
B)
Peristalsis
done
clear
C)
Systole of the ventricles
done
clear
D)
Dilation of pupil of the eye
done
clear
View Answer play_arrow
question_answer 148) This is not a GMO.
A)
Btbrinjal
done
clear
B)
Golden rice
done
clear
C)
Tracy
done
clear
D)
Dolly
done
clear
View Answer play_arrow
question_answer 149) The site of Krebs cycle is
A)
cytoplasm
done
clear
B)
mitochondrial matrix
done
clear
C)
intermembrane space of mitochondria
done
clear
D)
rackers particles
done
clear
View Answer play_arrow
question_answer 150) Which is the cutting organ in the mouth parts of cockroach?
A)
Labium
done
clear
B)
Maxillary palp
done
clear
C)
Mandible
done
clear
D)
Labrum
done
clear
View Answer play_arrow
question_answer 151) If this enzyme were to be absent in our small intestine, digestion of proteins in our body would be severely affected.
A)
Pancreatic amylase
done
clear
B)
Maltase
done
clear
C)
Lipase
done
clear
D)
Enter kinase
done
clear
View Answer play_arrow
question_answer 152) The frequency of heart beat in our body is maintained by
A)
AV Node
done
clear
B)
SA Node
done
clear
C)
Node of Ranvier
done
clear
D)
Chordae tendineae
done
clear
View Answer play_arrow
question_answer 153) Hypothalamus of the brain is not involved in this function.
A)
Sleep-wake cycle
done
clear
B)
Osmoregulation and thirst
done
clear
C)
Temperature control
done
clear
D)
Accuracy of muscular movement
done
clear
View Answer play_arrow
question_answer 154) The Hardy -Wemberg principle cannot operate If
A)
the population is very large
done
clear
B)
frequent mutations occur in the population
done
clear
C)
the population has no chance of interaction with other populations
done
clear
D)
free interbreeding occurs among all members of the population
done
clear
View Answer play_arrow
question_answer 155) The adult animal in this phylum is radially symmetrical; but its larva exhibits bilateral symmetry.
A)
Echinodermata
done
clear
B)
Coelenterata
done
clear
C)
Arthropoda
done
clear
D)
Protozoa
done
clear
View Answer play_arrow
question_answer 156) Identify the sense codon from the following.
A)
UGA
done
clear
B)
AUG
done
clear
C)
UAG
done
clear
D)
UAA
done
clear
View Answer play_arrow
question_answer 157) Select a suitable name for the following process. \[{{C}_{6}}{{H}_{12}}{{O}_{6}}+2ADP+2Pi\to 2{{C}_{2}}{{H}_{5}}OH+2ATP+2C{{O}_{2}}\uparrow \]
A)
Alcoholic fermentation
done
clear
B)
Photorespiration
done
clear
C)
Lactate fermentation
done
clear
D)
Aerobic respiration
done
clear
View Answer play_arrow
question_answer 158) The condition of erythroblastosis foetalis occurs only when
A)
the husband is \[R{{h}^{+}}\] and wife is \[R{{h}^{-}}\]
done
clear
B)
the husband is \[R{{h}^{-}}\]and wife is \[R{{h}^{+}}\]
done
clear
C)
the mother is \[R{{h}^{+}}\]and foetus is \[R{{h}^{-}}\]
done
clear
D)
the mother is \[R{{h}^{-}}\] and foetus is \[R{{h}^{+}}\]
done
clear
View Answer play_arrow
question_answer 159) This is a no biodegradable pollutant.
A)
Sewage
done
clear
B)
Sulphur dioxide
done
clear
C)
Oxides of nitrogen
done
clear
D)
Lead vapour
done
clear
View Answer play_arrow
question_answer 160) The time for optimum chances of conception in a woman is ----- starting from the day of menstruation.
A)
1st day
done
clear
B)
4th day
done
clear
C)
14th day
done
clear
D)
26th day
done
clear
View Answer play_arrow
question_answer 161) The fourth cleavage plane during development of frogs egg is
A)
double meridional
done
clear
B)
single meridional
done
clear
C)
single latitudinal
done
clear
D)
double latitudinal
done
clear
View Answer play_arrow
question_answer 162) Which of the following parts of the vertebrate body arises from the mesoderm?
A)
Spinal cord
done
clear
B)
Bony skeleton
done
clear
C)
Epidermis
done
clear
D)
Lens of the eye
done
clear
View Answer play_arrow
question_answer 163) Point out the correct method of showing scientific name of coconut palm derived by binomial nomenclature.
A)
Cocos nucifera
done
clear
B)
Cocos Nucifera
done
clear
C)
cocos Nucifera
done
clear
D)
cocos nucifera
done
clear
View Answer play_arrow
question_answer 164) Find out the wrong statement about angiosperm roots.
A)
Cuticle is absent in young stages.
done
clear
B)
The apex is protected by root cap.
done
clear
C)
Vascular bundles are collateral.
done
clear
D)
Xylem is centripetal in growth in the young roots.
done
clear
View Answer play_arrow
question_answer 165) The given figure shows the floral diagram of a flower. Which of the following descriptions of the flower matches the floral diagram?
A)
Heterochlamydeous, gamopetalous, pentamerous and bisexual
done
clear
B)
Heterochlamydeous, gamopetalous, tetramerous and bisexual
done
clear
C)
Homochlamydeous, polypetalous, pentamerous and bisexual
done
clear
D)
Homochlamydeous, gamopetalous, tetramerous and unisexual
done
clear
View Answer play_arrow
question_answer 166) An interconnecting membranous network of the cell composed of vesicles, flattened sacs and tubules is
A)
nucleus
done
clear
B)
mitochondrion
done
clear
C)
endoplasmic reticulum
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 167) Read the statements given below and identify the incorrect statement.
A)
Scientific names are used all over the world.
done
clear
B)
Scientific names are often descriptive and tell us some important character of an organism.
done
clear
C)
Scientific names indicate relationship between species.
done
clear
D)
Scientific names favour multiple naming for the same kind of an organism.
done
clear
View Answer play_arrow
question_answer 168) The lac operon is turned on when allolactose molecules bind to
A)
promoter site
done
clear
B)
operator site
done
clear
C)
mRNA
done
clear
D)
represser protein
done
clear
View Answer play_arrow
question_answer 169) Fearing that the child to be born may have a genetic disorder, a couple goes to a doctor. Which one of the following techniques is likely to be suggested by the doctor to cure the genetic disorder?
A)
Hybridoma technology
done
clear
B)
Gene therapy
done
clear
C)
r DNA technology ,
done
clear
D)
Embryo transfer
done
clear
View Answer play_arrow
question_answer 170) Select the group having only buffalo breeds of India from the following.
A)
Surti, Mehsana, Murrah, Nagpuri
done
clear
B)
Mehsana, Murrah, Nagpuri, Haryana
done
clear
C)
Murrah, Nagpuri, Haryana, Ongole
done
clear
D)
Nagpuri, Haryana, Ongole, Sindhi
done
clear
View Answer play_arrow
question_answer 171) With regard to the ABO blood typing system, if a man who has type B blood and a woman who has type 0 blood were to have children, what blood types could the children have?
A)
A or 0
done
clear
B)
B or 0
done
clear
C)
AB or 0
done
clear
D)
. A, B, AB or 0
done
clear
View Answer play_arrow
question_answer 172) Secretin and cholecystokinin are the hormones secreted in
A)
pyloric stomach
done
clear
B)
duodenum
done
clear
C)
ileum
done
clear
D)
oesophagus
done
clear
View Answer play_arrow
question_answer 173) Carbon dioxide is called a greenhouse gas, because
A)
it is involved in photosynthesis
done
clear
B)
it emits light
done
clear
C)
it traps infrared radiations
done
clear
D)
it traps ultraviolet radiations
done
clear
View Answer play_arrow
question_answer 174) A fruit that develops from a single flower with a syncarpous pistil is
A)
simple fruit
done
clear
B)
aggregate fruit
done
clear
C)
multiple fruit
done
clear
D)
pseudo carp
done
clear
View Answer play_arrow
question_answer 175) The volume of blood that enters into the aorta with each ventricular systole is called
A)
cardiac cycle
done
clear
B)
stroke volume
done
clear
C)
cardiac output
done
clear
D)
vital capacity
done
clear
View Answer play_arrow
question_answer 176) The chromosomal complement of individuals with Turners syndrome is
A)
\[44A+XX\]
done
clear
B)
\[44A+XY\]
done
clear
C)
\[44A+XO\]
done
clear
D)
\[44A+XXY\]
done
clear
View Answer play_arrow
question_answer 177) Choose the mismatched pair from the following.
A)
[a] Insulin - Gluconeogenesis
done
clear
B)
[b] Glucagon - Glycogenolysis
done
clear
C)
[c] Oxytocin - Contraction of uterine muscles
done
clear
D)
[d] Prolactin - Milk production in mammary glands
done
clear
View Answer play_arrow
question_answer 178) Which one of the following is not a wildlife conservation project?
A)
Project Dodo
done
clear
B)
Project Indian Bustard
done
clear
C)
Project Tiger
done
clear
D)
- Project Hangul
done
clear
View Answer play_arrow
question_answer 179) Visible expression of the genetic phenomenon of crossing over is called
A)
recombination
done
clear
B)
condensation
done
clear
C)
chiasmata
done
clear
D)
spiralization
done
clear
View Answer play_arrow
question_answer 180) 3 AAA TGC GCG ATA 5 is the sequence of nucleotides on a gene; after transcription the /77RNA formed against it and the sequence of bases in the corresponding binding anticodons will be
A)
\[5\text{ }UUU\text{ }ACG\text{ }CGC\text{ }UAU\text{ }3\]and \[3AAA-UGC-GCG-AUA\text{ }5\]
done
clear
B)
\[5UAU\text{ }CGC\text{ }GCA\text{ }UUU3\]and \[3AUA-GCG-CGU-AAA\text{ }5\]
done
clear
C)
\[5UUU\text{ }ACC\text{ }TUG\text{ }UAU\text{ }3\]and \[3AAA-UGG-UAC-AUA\text{ }5\]
done
clear
D)
\[5UAU\text{ }GUT\text{ }CCA\text{ }UUU\text{ }3\]and \[3AUA-CAU-GGU-AAA\text{ }5\]
done
clear
View Answer play_arrow