question_answer 1) If \[{{a}_{r}}\] and \[{{a}_{t}}\] represent radial and tangential accelerations, the motion of a particle will be uniformly circular if :
A)
\[{{a}_{r}}=\]and \[{{a}_{t}}=0\]
done
clear
B)
\[{{a}_{r}}=0\,\]but \[{{a}_{t}}\ne 0\,\]
done
clear
C)
\[{{a}_{r}}\ne 0\,\] but\[{{a}_{t}}=0\,\]
done
clear
D)
\[{{a}_{r}}\ne 0\,\]
done
clear
View Answer play_arrow
question_answer 2) Radius of orbit of satellite of earth is R. Its kinetic energy is proportional to :
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{\sqrt{R}}\]
done
clear
C)
\[R\]
done
clear
D)
\[\frac{1}{{{R}_{3/2}}}\]
done
clear
View Answer play_arrow
question_answer 3) The radius R of the soap bubble is doubled under isothermal condition. If T be the surface tension of soap bubble, the required surface energy in doing so is given by :
A)
\[32\pi {{R}^{2}}T\]
done
clear
B)
\[24\pi {{R}^{2}}T\]
done
clear
C)
\[8\pi {{R}^{2}}T\]
done
clear
D)
\[4\pi {{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 4) Mercury boils at \[367{}^\circ C\]. However, mercury thermometers are made such that they can measure temperature upto \[500{}^\circ C\]. This is done by :
A)
maintaining vacuum above mercury column in the stem of the thermometer
done
clear
B)
filling nitrogen gas at high pressure above the mercury column.
done
clear
C)
filling oxygen gas at high pressure above the mercury column
done
clear
D)
filling nitrogen gas at low pressure above the mercury column
done
clear
View Answer play_arrow
question_answer 5) Two similar coils are kept mutually perpendicular such that their centres coincide. At the centre, find the ratio of the magnetic field due to one coil and the resultant magnetic field through both coils, if the same current is flown :
A)
\[1:\sqrt{2}\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:2\]
done
clear
D)
\[\sqrt{3}:1\]
done
clear
View Answer play_arrow
question_answer 6) A prism of refractive index \[\sqrt{2}\] has a refracting angle of \[60{}^\circ C\]. At what angle a ray must be incident on it so that it suffers a minimum deviation?
A)
\[45{}^\circ C\]
done
clear
B)
\[60{}^\circ C\]
done
clear
C)
\[90{}^\circ C\]
done
clear
D)
\[180{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 7) A cone filled with water is revolved in a vertical circle of radius 4 m and the water does not fall down. What must be the maximum period of revolution?
A)
2 sec
done
clear
B)
4 sec
done
clear
C)
1 sec
done
clear
D)
6 sec
done
clear
View Answer play_arrow
question_answer 8) A transparent cube of 15cm edge contains a small air bubble. Its apparent depth when viewed through one face is 6 cm and when viewed through the opposite face is 4 cm. Then the refractive index of the material of the cube is :
A)
2.0
done
clear
B)
2.5
done
clear
C)
1.6
done
clear
D)
1.5
done
clear
View Answer play_arrow
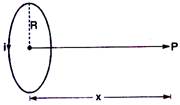
question_answer 9)
A coil having N turns carries a current as shown in the figure. The magnetic field intensity at point P is :
A)
\[\frac{{{\mu }_{0}}Ni{{R}^{2}}}{2({{R}^{2}}+{{x}^{2}})3/2}\]
done
clear
B)
\[\frac{{{\mu }_{0}}Ni}{2R}\]
done
clear
C)
\[\frac{{{\mu }_{0}}Ni{{R}^{2}}}{{{(R+x)}^{2}}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 10) A capacitor is connected to a cell of emf E having some internal resistance r. The potential difference across the :
A)
cell is < E
done
clear
B)
cell is E
done
clear
C)
capacitor is > E
done
clear
D)
capacitor is < E
done
clear
View Answer play_arrow
question_answer 11) The length, breadth and thickness of a block are given by \[l=12\,\,\text{cm,}\,\text{b=6}\] cm, and t=2.45 cm- The volume of the block according to the idea of significant figures should be :
A)
\[1\times {{10}^{2}}c{{m}^{3}}\]
done
clear
B)
\[2\times {{10}^{2}}c{{m}^{3}}\]
done
clear
C)
\[1.763\times {{10}^{2}}c{{m}^{3}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 12) Five particles of mass 2 kg are attached to the rim of a circular disc of radius m and negligible mass. Moment of inertia of the system about the axis passing through the centre of the disc and perpendicular to its plane is:
A)
1 kgm2
done
clear
B)
0.1 kg m2
done
clear
C)
2kgm2
done
clear
D)
0.2kgm2
done
clear
View Answer play_arrow
question_answer 13) The radius of the convex surface of plano-convex lens is 20 cm and me refractive index of the material of the lens is 1.5. The focal length is:
A)
30 cm
done
clear
B)
50 cm
done
clear
C)
20 cm
done
clear
D)
40 cm
done
clear
View Answer play_arrow
question_answer 14) An ice-cube of density 900 kg/m is floating in water of density n 1000 kg/m. The percentage of volume of ice-cube outside the water is :
A)
20%
done
clear
B)
35%
done
clear
C)
10%
done
clear
D)
25%
done
clear
View Answer play_arrow
question_answer 15) A sphere of diameter 0.2 m and mass kg is rolling on an inclined plane with velocity v=0.5 m/s. The kinetic energy of the sphere is :
A)
0.1 J
done
clear
B)
0.3 J
done
clear
C)
0.5 J
done
clear
D)
0.42 J
done
clear
View Answer play_arrow
question_answer 16) An electron moves at right angle to a magnetic field of \[1.5\times {{10}^{-2}}\] tesla with a speed of \[6\times {{10}^{7}}\] m/s. If the specific charge of the electron is \[1.7\times {{10}^{11}}\] coulomb/kg, the radius of the circular path will be:
A)
2.9 cm
done
clear
B)
3.9 cm
done
clear
C)
2.35 cm
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 17) If work function of a metal is 4.2 eV, the cut off wave length is :
A)
\[8000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[7000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1472\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2950\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 18) A particle is executing the motion \[x=a\,\cos (\omega t-\theta ).\]The maximum velocity of the particle is :
A)
\[a\,\omega \,\cos \theta \]
done
clear
B)
\[a\,\omega \,\]
done
clear
C)
\[a\,\omega \,\sin \theta \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) A particle is executing two different simple harmonic motions, mutually perpendicular, of different amplitudes and having phase difference of \[\pi /2\] The path of the particle will be :
A)
circular
done
clear
B)
straight line
done
clear
C)
parabolic
done
clear
D)
elliptical
done
clear
View Answer play_arrow
question_answer 20) Equations of motion in the same direction are given by : \[{{y}_{1}}-2a\sin (\omega t-kx)\] \[{{y}_{2}}-2a\sin (\omega t-kx\theta )\] The amplitude of the medium particle will be :
A)
\[2a\,\,\cos \,\theta \]
done
clear
B)
\[\sqrt{2a}\,\,\cos \,\theta \]
done
clear
C)
\[4a\,\,\cos \,\theta /2\]
done
clear
D)
\[\sqrt{2a}\,\,\cos \,\theta /2\]
done
clear
View Answer play_arrow
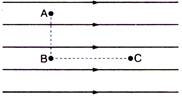
question_answer 21)
Figure shows three points A, B and C in a region of uniform electric field \[\mathbf{\vec{E}}.\]The line AB is perpendicular and BC is parallel to the field lines. Then which of the following holds good?
A)
\[{{V}_{A}}={{V}_{B}}={{V}_{C}}\]
done
clear
B)
\[{{V}_{A}}={{V}_{B}}>{{V}_{C}}\]
done
clear
C)
\[{{V}_{A}}={{V}_{B}}<{{V}_{C}}\]
done
clear
D)
\[{{V}_{A}}={{V}_{B}}>{{V}_{C}}\]
done
clear
View Answer play_arrow
question_answer 22)
The potential difference between points A and B is :
A)
\[\frac{20}{7}V\]
done
clear
B)
\[\frac{40}{7}V\]
done
clear
C)
\[\frac{10}{7}V\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 23) A short linear object of length b lies along the axis of a concave mirror of focal length \[f\] at a distance\[u\] from the pole of the mirror, what is the size of image?
A)
\[\left( \frac{f}{u-f} \right)b\]
done
clear
B)
\[{{\left( \frac{f}{u-f} \right)}^{2}}b\]
done
clear
C)
\[\left( \frac{f}{u-f} \right)b2\]
done
clear
D)
\[\left( \frac{f}{u-f} \right)\]
done
clear
View Answer play_arrow
question_answer 24) A closed organ pipe and an open organ pipe are tuned to the same fundamental frequency. What is the ratio of their lengths?
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
2 : 3
done
clear
D)
4 : 3
done
clear
View Answer play_arrow
question_answer 25) Regarding a semiconductor which one of the following is wrong?
A)
There are no free electrons at room temperature
done
clear
B)
There are no free electrons at OK
done
clear
C)
The number of free electrons increases with rise of temperature
done
clear
D)
The charge carriers are electrons and holes
done
clear
View Answer play_arrow
question_answer 26) A steel scale measures the length of a copper wire as 80.0 cm, when both are at 20°C, the calibration temperature for the scale. What would the scale read for the length of the wire when both are at 40°C? Given: \[\alpha \] for steel \[=11\times {{10}^{-6}}\] per °C and a for \[Cu=17\times {{10}^{-6}}\]per °C:
A)
80.0096 cm
done
clear
B)
80.0272 cm
done
clear
C)
1 cm
done
clear
D)
25.2 cm
done
clear
View Answer play_arrow
question_answer 27) A tank is filled with water upto height H. When a hole is made at a distance h below the level of water, what will be the horizontal range of water jet?
A)
\[2\sqrt{h(H-h)}\]
done
clear
B)
\[4\sqrt{h(H+h)}\]
done
clear
C)
\[4\sqrt{h(H-h)}\]
done
clear
D)
\[2\sqrt{h(H+h)}\]
done
clear
View Answer play_arrow
question_answer 28) A raft of wood of mass 120 kg float. in water. The weight that can be put on the raft to make it just sink, should be\[({{d}_{raft}}=600kg/{{m}^{3}}):\]
A)
80 kg
done
clear
B)
50 kg
done
clear
C)
60 kg
done
clear
D)
30 kg
done
clear
View Answer play_arrow
question_answer 29) Nuclear fusion is common to the pair s :
A)
thermonuclear reactor, uranium based nuclear reactor
done
clear
B)
energy production in sun, uranium based nuclear reactor
done
clear
C)
energy production in sun/ hydrogen bomb
done
clear
D)
disintegration of heavy nuclei, hydrogen bomb
done
clear
View Answer play_arrow
question_answer 30) Which of the following statements is true for an n-type semi-conductor?
A)
The donor level lies closely below the bottom of the conduction band
done
clear
B)
The donor level lies closely above the top of the valence band
done
clear
C)
The donor level lies at the halfway mark of the forbidden energy gap
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 31) The minimum wavelength of X-rays emitted by X-ray tube is 0.4125 A. The accelerating voltage is :
A)
30 kV
done
clear
B)
50 kV
done
clear
C)
80 kV
done
clear
D)
60 kV
done
clear
View Answer play_arrow
question_answer 32) A monoatomic gas supplied the heat \[Q\] very slowly keeping the pressure constant. The work done by the gas will be :
A)
\[\frac{2}{3}Q\]
done
clear
B)
\[\frac{3}{5}Q\]
done
clear
C)
\[\frac{2}{5}Q\]
done
clear
D)
\[\frac{1}{5}Q\]
done
clear
View Answer play_arrow
question_answer 33)
The refractive index of the material of the prism and liquid are 1.56 and 1.32 respectively. What will be the value of \[\theta \] for the following refraction?
A)
\[\sin \theta \ge \frac{13}{11}\]
done
clear
B)
\[\sin \theta \ge \frac{11}{13}\]
done
clear
C)
\[\sin \theta \ge \frac{\sqrt{3}}{2}\]
done
clear
D)
\[\sin \theta \ge \frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 34) The temperature of the black body Increases from T to 2T. The factor by which the rate of emission will increase, is:
A)
4
done
clear
B)
2
done
clear
C)
16
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 35) A police jeep is chasing with velocity of 45 km/h, a thief in another jeep moving with velocity 153 km/h. Police fires a , bullet with muzzle velocity of 180 m/s. The velocity with which it will strike the car of the thief, is :
A)
150 m/s
done
clear
B)
27 m/s
done
clear
C)
450 m/s
done
clear
D)
250 m/s
done
clear
View Answer play_arrow
question_answer 36) In a sinusoidal wave. the time required for a particular point to move from maximum displacement to zero displacement is 0-17 sec. The frequency of the wave is :
A)
1.47 Hz
done
clear
B)
2.94 Hz
done
clear
C)
0.73 Hz
done
clear
D)
0.36 Hz
done
clear
View Answer play_arrow
question_answer 37) An LC circuit is in the state of resonance. If \[C=0.1\mu F\]and \[L=0.25\] H, neglecting ohmic resistance of circuit, what is the frequency of oscillations?
A)
1007 Hz
done
clear
B)
100 Hz
done
clear
C)
109 Hz
done
clear
D)
500 Hz
done
clear
View Answer play_arrow
question_answer 38) A person who can see things most clearly at a distance of 10 cm, requires spectacles to enable to see clearly things at a distance of 30 cm. What should be the focal length of the spectacles?
A)
15 cm (concave)
done
clear
B)
15 cm (convex)
done
clear
C)
10 cm
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 39) The dimensional formula for Young's modulus is:
A)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
B)
\[[M{{L}^{0}}L{{T}^{-2}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 40) When temperature of an ideal gas is increased from 27°C to 227°C/ its rms speed is changed from 400 metre/sec to \[{{\upsilon }_{s.}}\] The \[{{\upsilon }_{s}}\] is :
A)
516 metre/sec
done
clear
B)
450 metre/sec
done
clear
C)
310 metre/sec
done
clear
D)
746 metre/sec
done
clear
View Answer play_arrow
question_answer 41) A bar magnet of magnetic moment \[\mathbf{\vec{M}}\] is placed in the magnetic field \[\mathbf{\vec{B}}\] The Torque acting on the magnet is :
A)
\[\mathbf{\vec{M}\times \vec{B}}\]
done
clear
B)
\[\mathbf{\vec{M}\times \vec{B}}\]
done
clear
C)
\[\frac{1}{2}\mathbf{\vec{M}\times \vec{B}}\]
done
clear
D)
\[\mathbf{\vec{M}}+\mathbf{\vec{B}}\]
done
clear
View Answer play_arrow
question_answer 42) A capacitor of capacitance 6 \[\mu F\] is charged upto 100 volt. The energy stored in the capacitor is :
A)
0.6 joule
done
clear
B)
0.06 joule
done
clear
C)
0.03 joule
done
clear
D)
0.3 joule
done
clear
View Answer play_arrow
question_answer 43) The radius of orbit of a planet is two times that of the earth. The time period of planet is :
A)
4.2 T
done
clear
B)
2.8 T
done
clear
C)
5.6 T
done
clear
D)
8.4 T
done
clear
View Answer play_arrow
question_answer 44) A body falls from a height h=200 m. The ratio of distance travelled in each sec, during t=0 to r=6 second of the journey is :
A)
1 : 4 : 9
done
clear
B)
1 : 2 : 4
done
clear
C)
1 : 3 : 5.
done
clear
D)
1 : 2 : 3
done
clear
View Answer play_arrow
question_answer 45) To make the frequency double of a spring oscillator, we have to :
A)
reduce the mass to one fourth
done
clear
B)
quardruple the mass
done
clear
C)
double the mass
done
clear
D)
half the mass
done
clear
View Answer play_arrow
question_answer 46) A particle executing SHM has amplitude m and frequency 60 Hz. The maximum acceleration of particle is :
A)
\[60{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
B)
\[80{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
C)
\[120{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
D)
\[144{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 47) When a wave travels in a medium the particles displacement is given by the equation\[y=0.03\] sin \[\pi \]\[(2t-0.01x),\] where \[x\] and \[y\] are in metre and t second. The wavelength of the wave is:
A)
200 m
done
clear
B)
100 m
done
clear
C)
20 m
done
clear
D)
10 m
done
clear
View Answer play_arrow
question_answer 48) If 300 ml of a gas at 27° C is cooled to 7° C at constant pressure, then its final volume will be :
A)
540 ml
done
clear
B)
350 ml
done
clear
C)
280 ml
done
clear
D)
135 ml
done
clear
View Answer play_arrow
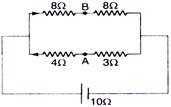
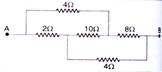
question_answer 49)
Find the equivalent resistance between the points A and B :
A)
\[2\Omega \]
done
clear
B)
\[4\Omega \]
done
clear
C)
\[8\Omega \]
done
clear
D)
\[16\Omega \]
done
clear
View Answer play_arrow
question_answer 50) When sound waves travel from air to water, which one of the following remains constant?
A)
Time period
done
clear
B)
Frequency
done
clear
C)
Velocity
done
clear
D)
Wavelength
done
clear
View Answer play_arrow
question_answer 51) A sphere rolls down an inclined plane of inclination \[\theta .\] What is the acceleration a the sphere reaches bottom?
A)
\[\frac{5}{7}g\,\sin \,\theta \]
done
clear
B)
\[\frac{3}{7}g\,\sin \,\theta .\]
done
clear
C)
\[\frac{2}{7}g\,\sin \,\theta .\]
done
clear
D)
\[\frac{2}{5}g\,\sin \,\theta .\]
done
clear
View Answer play_arrow
question_answer 52) In a bicycle the radius of rear wheel is twice the radius of front wheel. If \[{{r}_{f}}\] and \[{{r}_{r}}\] are the radius, \[{{\upsilon }_{f}}\]and \[{{\upsilon }_{r}}\] are the speeds of top most points of wheel, then :
A)
\[{{\upsilon }_{r}}=2{{\upsilon }_{f}}\]
done
clear
B)
\[{{\upsilon }_{f}}=2{{\upsilon }_{r}}\]
done
clear
C)
\[{{\upsilon }_{f}}={{\upsilon }_{r}}\]
done
clear
D)
\[{{\upsilon }_{f}}>{{\upsilon }_{r}}\]
done
clear
View Answer play_arrow
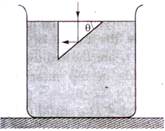
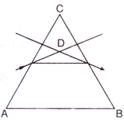
question_answer 53)
In the given figure, what is the angle of prism?
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 54) Moment of inertia of an object does not depend upon :
A)
mass of object
done
clear
B)
mass distribution
done
clear
C)
angular velocity
done
clear
D)
axis of rotation
done
clear
View Answer play_arrow
question_answer 55) A particle falls towards earth from infinity. Its velocity on reaching the earth would be :
A)
infinity
done
clear
B)
\[\sqrt{2gR}\]
done
clear
C)
\[2\sqrt{gR}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 56) Universal gas constant is :
A)
\[Cp/Cv\]
done
clear
B)
\[Cp-Cv\]
done
clear
C)
\[Cv+Cv\]
done
clear
D)
\[Cv-Cp\]
done
clear
View Answer play_arrow
question_answer 57) Two bodies of mass m and 4m have equal kinetic energy. What is the ratio of their momentum?
A)
1 : 4
done
clear
B)
1 : 2
done
clear
C)
1:1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
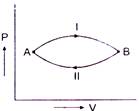
question_answer 58)
A gas at state A changes to state B through path I and II shown in figure. The change in internal energy is \[\Delta {{U}_{1}}\] and \[\Delta {{U}_{2}}\] respectively. Then :
A)
\[\Delta {{U}_{1}}>\Delta {{U}_{2}}\]
done
clear
B)
\[\Delta {{U}_{1}}<\Delta {{U}_{2}}\]
done
clear
C)
\[\Delta {{U}_{1}}=\Delta {{U}_{2}}\]
done
clear
D)
\[\Delta {{U}_{1}}=\Delta {{U}_{2}}=0\]
done
clear
View Answer play_arrow
question_answer 59) According to Kepler's law the time period of a satellite varies with its radius are as :
A)
\[{{T}^{2}}\propto {{R}^{3}}\]
done
clear
B)
\[{{T}^{2}}\propto {{R}^{2}}\]
done
clear
C)
\[{{R}^{2}}\propto (1/{{R}^{3}})\]
done
clear
D)
\[{{T}^{3}}\propto (1/{{R}^{2}})\]
done
clear
View Answer play_arrow
question_answer 60) A particle is moving in a circle with uniform speed \[\upsilon .\] In moving from a point to another diametrically opposite point:
A)
the momentum changes by \[m\upsilon \]
done
clear
B)
the momentum changes by \[2m\upsilon \]
done
clear
C)
the kinetic energy changes by \[(1/2)\,m{{\upsilon }^{2}}\]
done
clear
D)
the kinetic energy changes by\[\,m{{\upsilon }^{2}}\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following is not an ore of magnesium?
A)
Magnesite
done
clear
B)
Dolomite
done
clear
C)
Gypsum
done
clear
D)
Camalite
done
clear
View Answer play_arrow
question_answer 62) The solubility of\[S{{b}_{2}}{{S}_{3}}\]in water is \[1.0\times {{10}^{-5}}\]mol/litre at 298 K. What will be its solubility product?
A)
\[108\times {{10}^{-25}}\]
done
clear
B)
\[1.0\times {{10}^{-25}}\]
done
clear
C)
\[144\times {{10}^{-25}}\]
done
clear
D)
\[126\times {{10}^{-24}}\]
done
clear
View Answer play_arrow
question_answer 63) What will be the pH value of 0.05 M \[Ba{{(OH)}_{2}}\]solution?
A)
12
done
clear
B)
13
done
clear
C)
1
done
clear
D)
12.96
done
clear
View Answer play_arrow
question_answer 64) Which of the following will not react with\[NaOH\]?
A)
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
\[CH{{(CN)}_{3}}\]
done
clear
View Answer play_arrow
question_answer 65) Industrial name for\[{{H}_{2}}{{S}_{2}}{{O}_{7}}\]is:
A)
Pyrosulphuric acid
done
clear
B)
Marshall's acid
done
clear
C)
Oleum
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 66) \[{{I}_{2}}\]dissolves in\[KI\]solution due to the formation of:
A)
\[K{{I}_{2}}\]and F
done
clear
B)
\[{{K}^{+}},{{I}^{-}}\]and \[{{I}_{2}}\]
done
clear
C)
\[KI_{3}^{-}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 67) Water gas is:
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[CO+C{{O}_{2}}+C{{H}_{4}}\]
done
clear
C)
\[C{{O}_{2}}+{{N}_{2}}\]
done
clear
D)
\[CO+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 68) Chloroform, when kept open, is oxidized to:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[COC{{l}_{2}}\]
done
clear
C)
\[C{{O}_{2}},C{{l}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 69) lodoform test is not given by:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 70) In Me Arthur Forest method, silver is extracted from the solution of\[Na[Ag{{(CN)}_{2}}]\]by the use of:
A)
Fe
done
clear
B)
Mg
done
clear
C)
Cu
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 71) IUPAC name of \[C{{H}_{3}}C{{H}_{2}}C(Br)=\text{ }CHCl\]is:
A)
2-bromo-l-chloro butane
done
clear
B)
1-chloro-2-bromo-butene
done
clear
C)
3-chloro-2-bromo butene-2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 72) Which of the following elements never show positive oxidation number?
A)
O
done
clear
B)
Fe
done
clear
C)
Ga
done
clear
D)
F
done
clear
View Answer play_arrow
question_answer 73) Which of the following is anhydride of perchloric acid?
A)
\[C{{l}_{2}}{{O}_{7}}\]
done
clear
B)
\[C{{l}_{2}}{{O}_{5}}\]
done
clear
C)
\[C{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[HClO\]
done
clear
View Answer play_arrow
question_answer 74) Quantitative measurement of nitrogen in an organic compound is done by the method:
A)
Berthelot method
done
clear
B)
Belstein method
done
clear
C)
Lassaigne test
done
clear
D)
Kjheldahl method
done
clear
View Answer play_arrow
question_answer 75) Benedict's solution is not reduced by:
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
glucose
done
clear
D)
acetic anhydride
done
clear
View Answer play_arrow
question_answer 76) Which of the following sulphides is yellow in colour?
A)
\[Cus\]
done
clear
B)
\[CdS\]
done
clear
C)
\[ZnS\]
done
clear
D)
\[CoS\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following element is a metalloid?
A)
Bi
done
clear
B)
Sn
done
clear
C)
Ge
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 78) Brown ring in the test of\[NO_{3}^{-}\]is formed due to the formation of:
A)
\[FeS{{O}_{4}}.\text{ }NO\]
done
clear
B)
\[[Fe{{(S{{O}_{4}})}_{2}}.\text{ }NO]{{H}_{2}}O\]
done
clear
C)
\[F{{C}_{2}}{{(S{{O}_{4}})}_{3}}.NO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 79) The vapour pressure will be lowest for:
A)
0.1 M sugar solution
done
clear
B)
\[0.1\text{ }M\text{ }KCl\]solution
done
clear
C)
\[0.1\text{ }M\text{ }Cu{{(N{{O}_{3}})}_{2}}\]solution
done
clear
D)
\[0.1\text{ }M\text{ }AgN{{O}_{3}}\]solution
done
clear
View Answer play_arrow
question_answer 80) Which of the following is a false statement?
A)
Fluorine is more electronegative than chlorine
done
clear
B)
Nitrogen has greater\[I{{E}_{1}}\]than oxygen
done
clear
C)
Lithium is amphoteric
done
clear
D)
Chlorine is an oxidising agent
done
clear
View Answer play_arrow
question_answer 81) What is the name of element with atomic number 105?
A)
Kurchatovium
done
clear
B)
Dubnium
done
clear
C)
Nobelium
done
clear
D)
Holmium
done
clear
View Answer play_arrow
question_answer 82) Which kind of fission is favoured by sunlight?
A)
Heterolytic fission
done
clear
B)
Homolytic fission
done
clear
C)
Both and
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 83) The reagent used in Gatterman Koch aldehyde synthesis is:
A)
\[Pb/BaS{{O}_{4}}\]
done
clear
B)
alkaline\[KMn{{O}_{4}}\]
done
clear
C)
acidic \[KMn{{O}_{4}}\]
done
clear
D)
\[CO+HCl\]
done
clear
View Answer play_arrow
question_answer 84) Which type of isomerism is shown by propanal and propanone?
A)
Functional group
done
clear
B)
Metamerism
done
clear
C)
Tautomerism
done
clear
D)
Chain isomerism
done
clear
View Answer play_arrow
question_answer 85) lonisation depends upon:
A)
pressure
done
clear
B)
volume
done
clear
C)
dilution
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 86) Which of the following oxide does not form acidic aqueous solution?
A)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[NO\]
done
clear
View Answer play_arrow
question_answer 87) Which of the following is a use of alum?
A)
Making explosives
done
clear
B)
Bleaching clothes
done
clear
C)
Water softening
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 88) The first ionisation potential is maximum for:
A)
B
done
clear
B)
N
done
clear
C)
O
done
clear
D)
Be
done
clear
View Answer play_arrow
question_answer 89) Which of the following salt does not get hydrolysed in water?
A)
\[KCl{{O}_{4}}\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[C{{H}_{3}}COONa\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 90) In the reaction: \[{{H}_{2}}+{{I}_{2}}\] \[2HI\] In a 2 litre flask 0.4 moles of each\[{{H}_{2}}\]and\[{{I}_{2}}\]are taken. At equilibrium 0.5 moles of HI are formed. What will be the value of equilibrium constant,\[{{K}_{c}}\]?
A)
20.2
done
clear
B)
25.4
done
clear
C)
0.284
done
clear
D)
11.1
done
clear
View Answer play_arrow
question_answer 91) Blood cells will remain as such in:
A)
hypertonic solution
done
clear
B)
hypotonic solution
done
clear
C)
isotonic solution
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 92) Which among the following elements have lowest value of\[I{{E}_{1}}\]?
A)
Pb
done
clear
B)
Sn
done
clear
C)
Si
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 93) \[KI\]and\[CuS{{O}_{4}}\]solution when mixed, give:
A)
\[Cu{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}+C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+Cu{{I}_{2}}+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 94) Which of the following is a Lewis base?
A)
\[NaOH\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 95) Wave nature of electrons was demonstrated by:
A)
Schrodinger
done
clear
B)
De-Broglie
done
clear
C)
Davisson and Garmer
done
clear
D)
Heisenberg
done
clear
View Answer play_arrow
question_answer 96) Distribution law was given by:
A)
Henry
done
clear
B)
Vant Hoff
done
clear
C)
Nemst
done
clear
D)
Ostwald
done
clear
View Answer play_arrow
question_answer 97) When Cu reacts with\[AgN{{O}_{3}}\]solution, the reaction takes place is:
A)
Oxidation of Cu
done
clear
B)
Reduction of Cu
done
clear
C)
Oxidation of Ag
done
clear
D)
Reduction of\[N{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Cetane is a compound which has very good ignition property. Chemically it is:
A)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{14}}C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}C{{(C{{H}_{2}})}_{11}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{17}}{{H}_{34}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 99) Aldol condensation will not occur in:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 100) Which of the following alcohol is used as beverage?
A)
Propanol
done
clear
B)
2-butanol
done
clear
C)
Methanol
done
clear
D)
Ethanol
done
clear
View Answer play_arrow
question_answer 101) The oxidation number of carbon in\[C{{H}_{2}}O\]is:
A)
-2
done
clear
B)
+2
done
clear
C)
0
done
clear
D)
+4
done
clear
View Answer play_arrow
question_answer 102) Which of the following reaction involves oxidation and reduction?
A)
\[NaBr+HCl\xrightarrow{{}}NaCl+HBr\]
done
clear
B)
\[HBr+AgN{{O}_{3}}\xrightarrow{{}}AgBr+HN{{O}_{3}}\]
done
clear
C)
\[{{H}_{2}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]
done
clear
D)
\[N{{a}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 103) The electrophile involved in the nitration of benzene is:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
\[NO\]
done
clear
D)
\[NO_{2}^{-}\]
done
clear
View Answer play_arrow
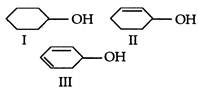
question_answer 104)
The correct order of ease of dehydration of following is:
A)
I > II > III
done
clear
B)
III > II > I
done
clear
C)
I > III > II
done
clear
D)
III > I > II
done
clear
View Answer play_arrow
question_answer 105) Fluorine is the best oxidising agent because it has:
A)
Highest electron affinity
done
clear
B)
Highest\[E_{red}^{o}\]red
done
clear
C)
highest\[E_{oxid}^{o}\]
done
clear
D)
lowest electron effinity
done
clear
View Answer play_arrow
question_answer 106) The bond order of\[O_{2}^{+}\]is the same as in:
A)
\[N_{2}^{+}\]
done
clear
B)
\[C{{N}^{-}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[N{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 107) When acetamide reacts with\[B{{r}_{2}}\]and caustic soda, then we get:
A)
acetic acid
done
clear
B)
bromoacetic acid
done
clear
C)
methyl amine
done
clear
D)
ethyl amine
done
clear
View Answer play_arrow
question_answer 108) Bleaching action of\[S{{O}_{2}}\]is due to its:
A)
oxidising property
done
clear
B)
acidic property
done
clear
C)
basic property
done
clear
D)
reducing property
done
clear
View Answer play_arrow
question_answer 109) When 12 is passed through\[KCl,\text{ }KF\]and\[KBr\] solutions:
A)
\[C{{l}_{2}}\]and\[B{{r}_{2}}\]are evolved
done
clear
B)
\[C{{l}_{2}}\]is evolved
done
clear
C)
\[C{{l}_{2}},B{{r}_{2}}\] and\[{{F}_{2}}\]are evolved
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 110) Which of the following dissolves in hot cone. \[NaOH\]solution?
A)
Fe
done
clear
B)
Zn
done
clear
C)
Cn
done
clear
D)
Ag
done
clear
View Answer play_arrow
question_answer 111) A gas can expand from 100 mL to 250 mL under a constant pressure of 2 atm. The work done by gas is:
A)
30.38 joule
done
clear
B)
25 joule
done
clear
C)
5 k joule
done
clear
D)
16 joule
done
clear
View Answer play_arrow
question_answer 112) If the r.m.s. speed of a gaseous molecule is\[x\] m/sec at a pressure P atm, then what will be the r.m.s. speed at a pressure 2P atm and constant temperature?
A)
\[x\]
done
clear
B)
\[2x\]
done
clear
C)
\[4x\]
done
clear
D)
\[x/4\]
done
clear
View Answer play_arrow
question_answer 113) Ionic mobility of\[A{{g}^{+}}\]is\[({{\lambda }_{A{{g}^{+}}}}5\times {{10}^{-1}}oh{{m}^{-1}}c{{m}^{2}}e{{q}^{-1}})\]:
A)
\[5.2\times {{10}^{-9}}\]
done
clear
B)
\[2.4\times {{10}^{-9}}\]
done
clear
C)
\[1.52\times {{10}^{-9}}\]
done
clear
D)
\[8.25\times {{10}^{-9}}\]
done
clear
View Answer play_arrow
question_answer 114) Which among the following is the strongest acid?
A)
\[HF\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 115) What is the general outer electronic configuration of the coinage metals?
A)
\[n{{s}^{2}}\text{ }n{{p}^{6}}\]
done
clear
B)
\[(n-1){{d}^{10}}\,n{{s}^{1}}\]
done
clear
C)
\[(n-1){{d}^{10}}\,n{{s}^{2}}\]
done
clear
D)
\[(n-1){{d}^{9}}\,n{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 116) How does the ionisation energy of 1st group elements vary?
A)
Increases down the group
done
clear
B)
Decreases down the group
done
clear
C)
Remains unchanged
done
clear
D)
Variation is not regular
done
clear
View Answer play_arrow
question_answer 117) What is the oxidation number of chlorine in\[CIO_{3}^{-}\]?
A)
+ 5
done
clear
B)
+ 3
done
clear
C)
+ 4
done
clear
D)
+ 2
done
clear
View Answer play_arrow
question_answer 118) What type of hybridisation takes place in the N atom of\[N{{H}_{3}}\]?
A)
\[s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[ds{{p}^{2}}\]
done
clear
D)
\[sp\]
done
clear
View Answer play_arrow
question_answer 119) What is the co-ordination number of CF in \[NaCl\]crystal?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 120) How many electrons are involved in oxidation of \[KMn{{O}_{4}}\] in basic medium?
A)
1
done
clear
B)
2
done
clear
C)
5
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 121) Thyroid deficiency infant leads to :
A)
hypothyroidism
done
clear
B)
myxedema
done
clear
C)
cretinism
done
clear
D)
thyrotoxicosis
done
clear
View Answer play_arrow
question_answer 122) Which of the following organs is not involved in the elicitation of immune response?
A)
Brain
done
clear
B)
Lymph nodes
done
clear
C)
Spleen
done
clear
D)
Thymus
done
clear
View Answer play_arrow
question_answer 123) Kuhne:
A)
discovered parathyroid
done
clear
B)
coined the term enzyme
done
clear
C)
coined the term gene
done
clear
D)
discovered enzyme
done
clear
View Answer play_arrow
question_answer 124) Mammalian prolactin is secreted by :
A)
adenohypophysis
done
clear
B)
neurohypophysis
done
clear
C)
adrenal cortex
done
clear
D)
adrenal medulla
done
clear
View Answer play_arrow
question_answer 125) Fever in malaria is due to :
A)
entry of sporozoites into blood capillaries
done
clear
B)
entry of merozoites into liver cells
done
clear
C)
release of merozoites from red blood cells
done
clear
D)
entry of cryptomerozoites into red blood cells
done
clear
View Answer play_arrow
question_answer 126) The origin of kidney and ureter in Rana tigrina is :
A)
all mesodermal
done
clear
B)
all endodermal
done
clear
C)
ectodermal and mesodennal
done
clear
D)
mesodermal and endodermal
done
clear
View Answer play_arrow
question_answer 127) Which of the following is not a case of epimorphosis?
A)
Formation of sperms from small clumps of cells
done
clear
B)
Regeneration of tail in a lizard
done
clear
C)
Replacement of severed arm in starfish
done
clear
D)
Replacement of limb in salamander
done
clear
View Answer play_arrow
question_answer 128) Which of the following is not found in Hydra?
A)
Epithelio-muscular cells
done
clear
B)
Cnidocyte
done
clear
C)
Choanocyte
done
clear
D)
Nerve cells
done
clear
View Answer play_arrow
question_answer 129) Which of the following pair is characterized by swollen lips, thick pigmented skin of hands and legs and irritability?
A)
Iodine - Goitre
done
clear
B)
Protein - Kwashiorkor
done
clear
C)
Thiamine - Beri-beri
done
clear
D)
Nicotinamide ? Pellagra
done
clear
View Answer play_arrow
question_answer 130) Which of the following structure in Pheretima posthuma plays the role of the liver of vertebrates?
A)
Calciferous glands
done
clear
B)
Gland cells
done
clear
C)
Chloragogen cells
done
clear
D)
Cliteller cells
done
clear
View Answer play_arrow
question_answer 131) Glomerular filtrate is :
A)
blood minus blood corpuscles and plasma protein
done
clear
B)
blood minus corpuscles
done
clear
C)
mixture of water, ammonia and corpuscles
done
clear
D)
urine
done
clear
View Answer play_arrow
question_answer 132) A condition in which body's internal environment remains relatively constant within limits is called :
A)
homeostasis
done
clear
B)
hemostasis
done
clear
C)
hematoma
done
clear
D)
haemopoiesis
done
clear
View Answer play_arrow
question_answer 133) Which of the following symmetry is found in adult sea-anemone?
A)
Radial
done
clear
B)
Biradial
done
clear
C)
Bilateral
done
clear
D)
Spherical
done
clear
View Answer play_arrow
question_answer 134) The parasphenoid bone in frog forms :
A)
base of cranium
done
clear
B)
floor of cranium
done
clear
C)
dorsal side of cranium
done
clear
D)
dorsolateral side of cranium
done
clear
View Answer play_arrow
question_answer 135) Body cavity of adult Ascaris is :
A)
haemocoel
done
clear
B)
amphicoel
done
clear
C)
pseudocoel
done
clear
D)
schizocoel
done
clear
View Answer play_arrow
question_answer 136) The peculiar pungent smell of cockroach is produced by the secretions of :
A)
pheromones
done
clear
B)
flame cells
done
clear
C)
abdominal glands
done
clear
D)
cervical glands
done
clear
View Answer play_arrow
question_answer 137) Intercellular communication in multicellular organism occurs through :
A)
digestive system only
done
clear
B)
respiratory system only
done
clear
C)
nervous system only
done
clear
D)
both nervous and endocrine system
done
clear
View Answer play_arrow
question_answer 138) The molecular structure of insulin was first described by :
A)
Korenberg
done
clear
B)
Swaminathan
done
clear
C)
Richardson
done
clear
D)
Sanger
done
clear
View Answer play_arrow
question_answer 139) Glycolysis occurs in :
A)
cytoplasm
done
clear
B)
nucleus
done
clear
C)
mitochondria
done
clear
D)
both 'a' and 'c'
done
clear
View Answer play_arrow
question_answer 140) Tongue is under control of :
A)
trigeminal nerve
done
clear
B)
facial nerve
done
clear
C)
automatic nervous system
done
clear
D)
glossopharyngeal nerve
done
clear
View Answer play_arrow
question_answer 141) Hypersecretion of growth hormone in the period of growth lead to :
A)
acromegaly
done
clear
B)
cushing syndrome
done
clear
C)
midgets
done
clear
D)
cretinism
done
clear
View Answer play_arrow
question_answer 142) Which of the following does not play a role in blood coagulation?
A)
Vitamin K.
done
clear
B)
Vitamin D
done
clear
C)
Calcium ions
done
clear
D)
Pibrinogen
done
clear
View Answer play_arrow
question_answer 143) The amount of bile released in- proportion to the amount of :
A)
fat in meal
done
clear
B)
protein in meal
done
clear
C)
carbohydrate in meal
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 144) Which of the following does not match?
A)
Pancreas - Glisson's capsule
done
clear
B)
Antigen - Antibody
done
clear
C)
Thyroid - Goitre
done
clear
D)
Enzyme - Substrate
done
clear
View Answer play_arrow
question_answer 145) The presence of arginase confirms that:
A)
urea cycle is operating
done
clear
B)
urea cycle may be operating
done
clear
C)
arginine is being converted into ornithine
done
clear
D)
arginine is being converted into citrulline
done
clear
View Answer play_arrow
question_answer 146) In a vertebrate which germ layer forms the skeleton muscles?
A)
Ectodenn
done
clear
B)
Endoderm
done
clear
C)
Mesoderm
done
clear
D)
Both 'a' and 'c'
done
clear
View Answer play_arrow
question_answer 147) Sudoriferous glands occur in :
A)
lung
done
clear
B)
kidney
done
clear
C)
skin
done
clear
D)
alimentary canal
done
clear
View Answer play_arrow
question_answer 148) Which of the following does not match?
A)
Muscular movement - ATP
done
clear
B)
Heart - pace-maker
done
clear
C)
Monocyte - haemoglobin
done
clear
D)
Nerve impulse - acetylcholine
done
clear
View Answer play_arrow
question_answer 149) Defect in ammo acid metabolism may results in :
A)
albinism
done
clear
B)
porphyria
done
clear
C)
Wilson's disease
done
clear
D)
phenylketonuria
done
clear
View Answer play_arrow
question_answer 150) Teeth of rabbits are :
A)
the codont
done
clear
B)
diphyodont
done
clear
C)
heterodont
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 151) Largest number of chloroplast is found in :
A)
palisade tissue
done
clear
B)
spongy tissue
done
clear
C)
transfusion tissue
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 152) A protein rich green alga is :
A)
Chlorella
done
clear
B)
Spirulina
done
clear
C)
Spirogyra
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 153) Growth rings are formed due to activity of :
A)
extrastelar cambium
done
clear
B)
intrastelar cambium
done
clear
C)
interstelar cambium
done
clear
D)
both 'b' and 'c'
done
clear
View Answer play_arrow
question_answer 154) In the sporogonium of which plant, columella is present?
A)
Penicillium
done
clear
B)
Spirogyra
done
clear
C)
Rhizopus
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 155) Rarely among angiosperms the pollen grains influenced the endosperm. This is called as :
A)
Metaxenia
done
clear
B)
Nemec phenomenon
done
clear
C)
Xenia
done
clear
D)
Mesogamy
done
clear
View Answer play_arrow
question_answer 156) Which of the following taxonomist described classification of plant kingdom in "Families of flowering plants"?
A)
Cronquist
done
clear
B)
Takhtajan
done
clear
C)
Benson
done
clear
D)
Hutchinson
done
clear
View Answer play_arrow
question_answer 157) A bryophyte which harbours a nitrogen fixing blue-green alga in its thallus is :
A)
Pogonatum
done
clear
B)
Riccia
done
clear
C)
Marchantia
done
clear
D)
Anthoceros
done
clear
View Answer play_arrow
question_answer 158) Phytochrome occurs in two forms. In which form it promotes the germination of seeds of some species?
A)
\[{{P}_{fr}}\]forms
done
clear
B)
\[{{P}_{r}}\]forms
done
clear
C)
Both forms
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 159) Which of the following is not a co-enzyme?
A)
NAD
done
clear
B)
NADP
done
clear
C)
FAD
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 160) Chiasmata are most appropriately observed in meiosis during:
A)
diakinesis
done
clear
B)
diplotene
done
clear
C)
metaphase II
done
clear
D)
pachytene
done
clear
View Answer play_arrow
question_answer 161) In case of \[{{C}_{4}}\]plants the acceptor of \[C{{O}_{2}}\]is :
A)
Phosphoglyceraldehyde
done
clear
B)
Ribulose monophosphate
done
clear
C)
Phosphoenol pyruvate
done
clear
D)
Ribulose diphosphate
done
clear
View Answer play_arrow
question_answer 162) Good soil is :
A)
which holds whole of the water that enters into it
done
clear
B)
which allows percolating the water slowly from it
done
clear
C)
which allows water to pass very quickly from it
done
clear
D)
which allows limited amount of water to retain into it
done
clear
View Answer play_arrow
question_answer 163) A metal ion involved in stomatal regulation is :
A)
iron
done
clear
B)
potassium
done
clear
C)
zinc
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 164) Starch is insoluble in water, yet it is accumulated in large quantities in potato tuber because :
A)
it is useful for storage
done
clear
B)
tubers respire slowly
done
clear
C)
starch is synthesized in tubers
done
clear
D)
translocated sucrose is polymerized here
done
clear
View Answer play_arrow
question_answer 165) The first step in dark reaction of photosynthesis is :
A)
formation of ATP
done
clear
B)
ionization of water
done
clear
C)
attachment of \[C{{O}_{2}}\]to a pentose sugar
done
clear
D)
excitement of electron of chlorophyll by a photon of light
done
clear
View Answer play_arrow
question_answer 166) Insect resistance transgenic cotton has been produced by inserting a piece of DNA from :
A)
an insect
done
clear
B)
a bacterium
done
clear
C)
a wild relative of cotton
done
clear
D)
a virus
done
clear
View Answer play_arrow
question_answer 167) The circinate vernation is the characteristic feature of ferns. It refers to :
A)
coiling of young leaves
done
clear
B)
arrangement of leaves on stem
done
clear
C)
attachment of son on leaves
done
clear
D)
heterophilly
done
clear
View Answer play_arrow
question_answer 168) Gymnosperms are called naked seed plants because these lack :
A)
cotyledon
done
clear
B)
endosperm
done
clear
C)
ovary wall
done
clear
D)
testa
done
clear
View Answer play_arrow
question_answer 169) Mutations which alter nudeotide sequence within a gene are :
A)
frame shift mutations
done
clear
B)
base pair substitutions
done
clear
C)
both 'a' and 'b'
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 170) Sugarcane show high efficiency of \[C{{O}_{2}}\]fixation because of :
A)
Calvin cycle
done
clear
B)
Hatch and Slack cycle
done
clear
C)
TCA cycle
done
clear
D)
greater sunlight
done
clear
View Answer play_arrow
question_answer 171) The plant ash is an indication of :
A)
organic matter of plant
done
clear
B)
waste product
done
clear
C)
mineral salts absorbed by plants
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 172) Which of the following fungi is found useful in the biological control of plant disease ?
A)
Penicillium notatum
done
clear
B)
Phytophthora parasitica
done
clear
C)
Mucor mucido
done
clear
D)
Trichoderma uiridae
done
clear
View Answer play_arrow
question_answer 173) Which organism was used by Beadle and Tatum to proposed one gene-one enzyme hypothesis?
A)
E. coli
done
clear
B)
Nostoc
done
clear
C)
Drosophila
done
clear
D)
Neurospora
done
clear
View Answer play_arrow
question_answer 174) When a tall plant with rounded seeds (TTRR) is crossed with a dwarf plant with wrinkled seeds (ttrr), the \[{{F}_{1}}\]generation consists of tall plants with rounded seeds. How many types of gametes an \[{{F}_{1}}\]plant would produce?
A)
One
done
clear
B)
Three
done
clear
C)
Four
done
clear
D)
Eight
done
clear
View Answer play_arrow
question_answer 175) Tetradynamous stamens are found in :
A)
Chrysanthemum
done
clear
B)
Zinnia
done
clear
C)
Poppy
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 176) Diadelphous stamens are the characteristic features of :
A)
Ranunculaceae
done
clear
B)
Fab ace ae
done
clear
C)
Poaceae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 177) Which is synthesized in \[{{G}_{1}}\]phase?
A)
DNA polymerase
done
clear
B)
Histones
done
clear
C)
Nucleolar DNA
done
clear
D)
Tubulin protein
done
clear
View Answer play_arrow
question_answer 178) Which of the following plant yields oil and fibre both ?
A)
Cocos nucifera
done
clear
B)
Eucalyptus
done
clear
C)
Brassica campestris
done
clear
D)
Euphorbia hirta
done
clear
View Answer play_arrow
question_answer 179) Capitulum inflorescence is found in :
A)
Compositae (Asteraceae)
done
clear
B)
Cruciferae (Brassicaceae)
done
clear
C)
Solanaceae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 180) Human immuno defidency virus causes :
A)
Acquired immuno deficiency syndrome
done
clear
B)
Anthrax
done
clear
C)
Tuberculosis
done
clear
D)
Polio
done
clear
View Answer play_arrow