question_answer 1)
The following is the truth table for : A B Y 0 0 1 1 0 1 0 1 1 1 1 0
A)
NAND
done
clear
B)
AND
done
clear
C)
XOR
done
clear
D)
NOT
done
clear
View Answer play_arrow
question_answer 2) In a full wave rectifier circuit operating from 50 Hz main frequency, the fundamental frequency in the ripple would be :
A)
25 Hz
done
clear
B)
50 Hz
done
clear
C)
70.7 Hz
done
clear
D)
100 Hz
done
clear
View Answer play_arrow
question_answer 3) The current gain a of a transistor is 0.9. The transistor is connected to common base configuration. What would be the change in collector current when base current changes by 4 mA?
A)
1.2 mA
done
clear
B)
12 mA
done
clear
C)
24 mA
done
clear
D)
36 mA
done
clear
View Answer play_arrow
question_answer 4) What is the name of the level formed due to impurity atom in p-type in the forbidden gap?
A)
Donor level
done
clear
B)
Acceptor level
done
clear
C)
Conduction level
done
clear
D)
Forbidden level
done
clear
View Answer play_arrow
question_answer 5) Which of the following is not true?
A)
All unit cells are primitive
done
clear
B)
FCC structure is a closed packed structure
done
clear
C)
A unit cell is primitive if it contains lattice points only at its corner
done
clear
D)
A lattice does not contain any atom or molecule
done
clear
View Answer play_arrow
question_answer 6) A radioactive element A decays into B with a half-life of 2 days. A fresh prepared sample of A has a mass of 12 g. What mass of A and B are there in the sample after 4 days?
A)
A = 3g, B = 9g
done
clear
B)
A = 6 g, B = 6 g
done
clear
C)
A = 12g, B = 0 g
done
clear
D)
A = 9g, B = 3g
done
clear
View Answer play_arrow
question_answer 7) How many \[\alpha \]-particle and \[\beta \]-particles are emitted when uranium nucleus \[_{92}^{238}U\]decays to lead nucleus \[_{82}^{206}Pb\]?
A)
\[\alpha \]= 6, \[\beta \]= 8
done
clear
B)
\[\alpha \]=10,\[\beta \]=8
done
clear
C)
\[\alpha \]= 8, \[\beta \]= 10
done
clear
D)
\[\alpha \]= 8,\[\beta \] =6
done
clear
View Answer play_arrow
question_answer 8) The angular speed of the electric in the nth orbit of Bohr hydrogen atom is :
A)
directly proportional to n
done
clear
B)
inversely proportional to \[\sqrt{n}\]
done
clear
C)
inversely proportional to n2
done
clear
D)
inversely proportional to n3
done
clear
View Answer play_arrow
question_answer 9) If the electron in hydrogen atom jumps from the third to second orbit, the wavelength of the emitted radiation in terms of Rydberg constant R is given by :
A)
\[\lambda =\frac{36}{5R}\]
done
clear
B)
\[\lambda =\frac{5R}{36}\]
done
clear
C)
\[\lambda =\frac{5}{R}\]
done
clear
D)
\[\lambda =\frac{R}{6}\]
done
clear
View Answer play_arrow
question_answer 10) When a monochromatic point source of light is at a distance of 0.2 m from a photocell, the cut-off voltage and the saturation current are respectively \[{{V}_{0}}=0.6\]volt and Js = 18.0 mA. If the same source is placed 0.6 m away from the photocell, then :
A)
stopping potential \[{{V}_{0}}=~0.2\] volt and saturation current J, = 18.0 mA
done
clear
B)
stopping potential \[{{V}_{0}}=0.6\] volt and saturation current Is = 18.0 mA
done
clear
C)
stopping potential \[{{V}_{0}}=0.6\] volt and saturation current Is = 2.0 mA
done
clear
D)
stopping potential \[{{V}_{0}}=2.0\] volt and saturation current Is = 2.0 mA
done
clear
View Answer play_arrow
question_answer 11) When light of wavelength 300 nm (nanometre) falls on a photoelectric emitter, photoelectrons are just liberated. For another emitter, however, light of 600 nm wavelength is sufficient for creating photoemission. What is the ratio of the work function of the two emitters?
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
4 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 12) In Millikans oil drop experiment, a charged drop of mass \[1.8\times {{10}^{-14}}kg\] is stationary between the plates. The distance between the plates 0.9 cm and potential difference between the plates is 2000 V. The number of electons on the oil drop is:
A)
10
done
clear
B)
5
done
clear
C)
50
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 13) In the experiment for the determination of\[\frac{e}{m}\] of electrons by the Thomson method, electric and magnetic fields are :
A)
parallel and both are perpendicular to the motion of the electron
done
clear
B)
both mutually perpendicular and parallel to the motion of electron
done
clear
C)
both mutually perpendicular and also perpendicular to the motion of electron
done
clear
D)
both mutually perpendicular and have no relation with motion of the electron
done
clear
View Answer play_arrow
question_answer 14) Blue colour of sky is due to :
A)
interference
done
clear
B)
scattering of light
done
clear
C)
dispersion of light
done
clear
D)
sun emits more of blue light
done
clear
View Answer play_arrow
question_answer 15) In Youngs double slit experiment distance between source is 1 mm and distance between the screen and source is 1 m. If the fringe width on the screen is 0.06 cm, then \[\lambda \] is :
A)
6000\[\overset{0}{\mathop{A}}\,\]
done
clear
B)
4000 \[\overset{0}{\mathop{A}}\,\]
done
clear
C)
1200 \[\overset{0}{\mathop{A}}\,\]
done
clear
D)
2400 \[\overset{0}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 16) Angle of minimum deviation for a prism of refractive index 1.5 is equal to the angle of the prism. The angle of the prism is : (given \[cos\text{ }41{}^\circ -24-36\text{ }=\text{ }0.75\]}
A)
\[82{}^\circ -{{49}^{}}-12\]
done
clear
B)
\[72{}^\circ -48-30\]
done
clear
C)
\[41{}^\circ -24-36\]
done
clear
D)
\[{{31}^{0}}-49-30\]
done
clear
View Answer play_arrow
question_answer 17) Two media having speeds of light \[2\times {{10}^{8}}m\text{ }:\] and \[2.4\times {{10}^{8}}m/s,\] are separated by a plane surface. What is the critical angle for a ray going from medium I to medium II?
A)
\[{{\sin }^{-1}}\left( \frac{5}{6} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{5}{12} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{1}{\sqrt{2}} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 18) When sunlight is incident on a prism, it produces a spectrum due to :
A)
interference of light
done
clear
B)
diffraction of light
done
clear
C)
total internal reflection
done
clear
D)
variation in speeds of different colours of light in the prism
done
clear
View Answer play_arrow
question_answer 19) The transverse nature of light is shown by :
A)
interference of light
done
clear
B)
diffraction of light
done
clear
C)
polarization
done
clear
D)
radiation spectrum of black body
done
clear
View Answer play_arrow
question_answer 20) An electromagnetic radiation of wavelength \[\lambda ,\] frequency v and propagating in air with velocity v is incident on a glass plate and is transmitted through. Which of the following statement is true for the wave inside the glass plate?
A)
The velocity v remains unchanged but\[\lambda ,\] changes
done
clear
B)
The frequency v and wavelength remain unchanged but v changes
done
clear
C)
The wavelength\[\lambda ,\]remains unchanged but velocity v changes
done
clear
D)
The frequency v remains unchanged but\[\lambda ,\] changes
done
clear
View Answer play_arrow
question_answer 21) The oscillating electric and magnetic vectors of an electromagentic wave in vacuum are oriented along :
A)
the same direction but differ in phase by \[90{}^\circ \]
done
clear
B)
the same direction and are in phase
done
clear
C)
mutually perpendicular directions and differ in phase by \[90{}^\circ \]
done
clear
D)
mutually perpendicular directions and are in phase
done
clear
View Answer play_arrow
question_answer 22) In an AC circuit the instantaneous values of emf and current aree = 200 sin 3001 volt and \[i=2\sin \left( 300t\frac{\pi }{3} \right)\] amp the average power consumed in watts is:
A)
200
done
clear
B)
100
done
clear
C)
50
done
clear
D)
400
done
clear
View Answer play_arrow
question_answer 23) A transformer is used to light a 140 W, 24 V lamp from 240 V AC mains. The current in the mains cable of 0.7 A. The efficiency of the transformer is:
A)
48%
done
clear
B)
63.8%
done
clear
C)
c) 83.3%
done
clear
D)
90%
done
clear
View Answer play_arrow
question_answer 24) A solenoid has 2000 turns wound over a length of 0.30 m. The area of its cross-section is \[1.2\times {{10}^{-3}}\text{ }{{m}^{2}}.\] Around its central section a coil of 300 turns is wound. If an initial current of 2 A in the solenoid is reversed in 0.25 s, the emf induced in the coil is:
A)
\[48\text{ }V\]
done
clear
B)
\[4.8\text{ }V\]
done
clear
C)
\[4.8\times {{10}^{-1}}V\]
done
clear
D)
\[4.8\times {{10}^{-2}}V\]
done
clear
View Answer play_arrow
question_answer 25) A copper ring is held horizontally and a bar magnet with its length along the axis of the ring is dropped through the ring. The acceleration of the falling magnet is :
A)
less than that due to gravity
done
clear
B)
equal to that due to gravity
done
clear
C)
more than that due to gravity
done
clear
D)
depend on the length of the magnet and diameter of the ring
done
clear
View Answer play_arrow
question_answer 26) The dimensional formula of Plancks constant (h)is:
A)
\[[M{{L}^{-2}}{{T}^{-3}}]\]
done
clear
B)
\[[M{{L}^{2}}{{V}^{-1}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{-2}}{{T}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 27) 27. A particle starts from rest and experiences constant acceleration for 6 s. If it travels a distance \[{{d}_{1}}\] in the first two seconds, a distance \[{{d}_{2}}\] in the next two seconds and a distance \[{{d}_{3}}\] in the last two seconds, then :
A)
\[{{d}_{1}}:\text{ }{{d}_{2}}:\text{ }{{d}_{3}}=\text{ }1:1:1\]
done
clear
B)
\[{{d}_{1}}:{{d}_{2}}:\text{ }{{d}_{3}}=\text{ }1\text{ }:\text{ }2:\text{ }3\]
done
clear
C)
\[{{d}_{1}}:\text{ }{{d}_{2}}:\text{ }{{d}_{3}}=\text{ }1:\text{ }3:\text{ }5\]
done
clear
D)
\[{{d}_{1}}:\text{ }{{d}_{2}}:\text{ }{{d}_{3}}=\text{ }1:\text{ }5:\text{ }9\]
done
clear
View Answer play_arrow
question_answer 28)
A particle originally at rest at the highest point of a smooth circle in a vertical plane, is gently pushed and starts sliding along the circle. It will leave the circle at a vertical distance h below the highest point such that:
A)
h=2R
done
clear
B)
\[h=\frac{R}{2}\]
done
clear
C)
h=R
done
clear
D)
\[h=\frac{R}{3}\]
done
clear
View Answer play_arrow
question_answer 29) An inclined plane has an inclination 0 with horizontal. A body of mass m rests on it. If the coefficient of friction between the body and the plane is n, then the minimum force that needs to be applied parallel to the inclined plane is :
A)
mg sin \[\theta \]
done
clear
B)
\[\mu mg\cos \theta \]
done
clear
C)
\[\mu mg\cos \theta +mg\sin \theta \]
done
clear
D)
\[\mu mg\cos \theta -mg\sin \theta \]
done
clear
View Answer play_arrow
question_answer 30) A 60 kg man stands on a spring scale in a lift. At some instant he finds that the scale reading has changed from 60 kg to 50 kg for a while and then comes again to 60 kg mark. What should he conclude?
A)
The lift was in constant motion upwards
done
clear
B)
The lift was in constant motion downwards
done
clear
C)
The lift while in motion suddenly stopped
done
clear
D)
The lift while in motion upwards suddenly stopped
done
clear
View Answer play_arrow
question_answer 31) A diatomic molecule is formed by two atoms which may be treated as mass points \[{{m}_{1}}\]and\[{{m}_{2}},\]joined by a massless rod of length r. Then the, moment of inertia of the molecule about an axis passing through the centre of mass and perpendicular to rod is :
A)
zero
done
clear
B)
\[({{m}_{1}}+{{m}_{2}}){{r}^{2}}\]
done
clear
C)
\[\left( \frac{{{m}_{1}}+{{m}_{2}}}{{{m}_{1}}{{m}_{2}}} \right){{r}^{2}}\]
done
clear
D)
\[\left( \frac{{{m}_{1}}{{m}_{2}}}{{{m}_{1}}+{{m}_{2}}} \right){{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 32) If the polar ice caps of earth melt, how long it affect the length of day?
A)
Length of day would remain unchanged
done
clear
B)
Length of day would increase
done
clear
C)
Length of day would decrease
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 33) A planet revolves in an elliptical orbit around the sun. The semi-major and semi-minor axes are a and b, then the period is given by :
A)
\[{{T}^{2}}\alpha {{b}^{3}}\]
done
clear
B)
\[{{T}^{2}}\alpha {{\left( \frac{a+b}{2} \right)}^{3}}\]
done
clear
C)
\[{{T}^{2}}\alpha {{a}^{3}}\]
done
clear
D)
\[{{T}^{2}}\alpha {{\left( \frac{a-b}{2} \right)}^{3}}\]
done
clear
View Answer play_arrow
question_answer 34) In a capillary tube of which the lower end dips in a liquid, the liquid rises to a height of 10 cm. If a capillary of the same bore is taken, whose length is 5 cm and dipped in liquid, then :
A)
a fountain of liquid will be obtain
done
clear
B)
the liquid will not rise in the rube at all
done
clear
C)
the liquid will rise upto the top and slowly ooze out of it
done
clear
D)
the liquid will rise to the top and will stay there
done
clear
View Answer play_arrow
question_answer 35) There relation between Y (Youngs modulus), K (bulk modulus) and n (shear modulus) is :
A)
\[\frac{9}{y}=\frac{1}{k}+\frac{3}{\eta }\]
done
clear
B)
\[\frac{1}{y}=\frac{1}{3\lambda }+\frac{1}{9k}\]
done
clear
C)
\[\frac{9}{y}=\frac{1}{\eta }+\frac{3}{k}\]
done
clear
D)
\[\frac{1}{\eta }=\frac{1}{k}+\frac{1}{y}\]
done
clear
View Answer play_arrow
question_answer 36) Solar radiation emitted by sun correspond to that emitted by black body at a temperature of 6000 K. Maximum intensity is emitted at wavelength of 4800\[\overset{0}{\mathop{A}}\,\] If the sun was to cool down from 6000 K to 3000 K, then the peak intensity of emitted radiation would occur at a wavelength :
A)
4300\[\overset{0}{\mathop{A}}\,\]
done
clear
B)
9600\[\overset{0}{\mathop{A}}\,\]
done
clear
C)
2400\[\overset{0}{\mathop{A}}\,\]
done
clear
D)
19200\[\overset{0}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 37) An ideal gas heat engine operates in a Carnot cycle between 227°C and 127°C. It absorbs \[6\times {{10}^{4}}\]cal at the higher temperature. The amount of heat converted into work is :
A)
\[4.8\times {{10}^{4}}cal\]
done
clear
B)
\[1.2\times {{10}^{4}}cal\]
done
clear
C)
\[3.5\times {{10}^{4}}cal\]
done
clear
D)
\[1.6\times {{10}^{4}}cal\]
done
clear
View Answer play_arrow
question_answer 38) A certain mass of gas at NTP is expanded to three times its volume under adiabatic conditions. The resulting temperature of gas will be : (y for gas is 1.40)
A)
\[273\times {{\left( \frac{1}{3} \right)}^{1.4}}\]
done
clear
B)
\[273\times {{(3)}^{0.4}}\]
done
clear
C)
\[273\times {{\left( \frac{1}{3} \right)}^{0.4}}\]
done
clear
D)
\[273\times {{(3)}^{1.4}}\]
done
clear
View Answer play_arrow
question_answer 39) If for a gas\[\frac{R}{{{C}_{v}}}=0.67,\] this gas is made up of molecules which are :
A)
monoatomic
done
clear
B)
diatomic
done
clear
C)
polyatomic
done
clear
D)
mixture of diatomic and polyatomic molecules
done
clear
View Answer play_arrow
question_answer 40) The velocity of a small ball of mass M and density \[{{d}_{1}}\] when dropped in a container filled with glycerine becomes constant after some time. If the density of glycerine is \[{{d}_{2}},\] the viscous force acting on the ball is :
A)
\[mg\left( 1-\frac{{{d}_{2}}}{{{d}_{1}}} \right)\]
done
clear
B)
\[mg\frac{{{d}_{1}}}{{{d}_{2}}}\]
done
clear
C)
\[mg({{d}_{1}}-{{d}_{2}})\]
done
clear
D)
\[mg{{d}_{1}}{{d}_{2}}\]
done
clear
View Answer play_arrow
question_answer 41) The length of an elastic spring is a metres when a force of 4 N is applied, and b metres when the 5N force is applied. Then the length of the spring when the 9 N force is applied is :
A)
a + b
done
clear
B)
9b - 9a
done
clear
C)
5b - 4a
done
clear
D)
4a - 5b
done
clear
View Answer play_arrow
question_answer 42) Moment of inertia of a uniform disc about a diameter is I. Its moment of inertia about an axis perpendicular to its plane and passing through a point on its rim will be :
A)
51
done
clear
B)
31
done
clear
C)
41
done
clear
D)
61
done
clear
View Answer play_arrow
question_answer 43) The escape velocity of a body from the surface of earth is 11.2 km/s. It is thrown up with a velocity 4 times this velocity of escape. The velocity of the body when it has escaped the gravitational pull of earth (neglecting presence of all other heavenly bodies) is :
A)
\[4\times 11.2km/s\]
done
clear
B)
\[\sqrt{15}\times 11.2km/s\]
done
clear
C)
zero
done
clear
D)
\[3\times 11.2\text{ }km/s\]
done
clear
View Answer play_arrow
question_answer 44) If a planet of given density were made larger (keeping its density unchanged) its force of attraction for an object on its surface would increase because of increased mass of the planet but would decrease because of larger separation between the centre of the planet and its surface. Which effect would dominate?
A)
Increase in mass
done
clear
B)
Increase in radius
done
clear
C)
Both affect the attraction equally
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 45)
Two balls each of mass m are placed on the vertices A and B of an equilateral triangle ABC of side 1 m. A ball of mass 2m is placed at vertex C. The centre of mass of this system from vertex A (located at origin) is :
A)
\[\left( \frac{1}{2}m,\frac{1}{2}m \right)\]
done
clear
B)
\[\left( \frac{1}{2}m,\sqrt{3}m \right)\]
done
clear
C)
\[\left( \frac{1}{2}m,\frac{\sqrt{3}}{4}m \right)\]
done
clear
D)
\[\left( \frac{\sqrt{3}}{4}m,\frac{\sqrt{3}}{4}m \right)\]
done
clear
View Answer play_arrow
question_answer 46) A particle moves along the x-axis from \[x={{x}_{1}}\]to \[x={{x}_{2}}\] under the action of a force given by F = 2x. Then the work done in the process is :
A)
zero
done
clear
B)
\[x_{2}^{2}-x_{1}^{2}\]
done
clear
C)
\[2{{x}_{2}}({{x}_{2}}-{{x}_{1}})\]
done
clear
D)
\[2{{x}_{1}}({{x}_{1}}-{{x}_{2}})\]
done
clear
View Answer play_arrow
question_answer 47) A body of mass 5 kg strikes another body of mass 2.5 kg initially at rest. The bodies after collision coalesce and begin to move as whole with a kinetic energy of 5 J. The kinetic energy of the first body before collision is :
A)
7.5 J
done
clear
B)
5 J
done
clear
C)
2.5 J
done
clear
D)
10 J
done
clear
View Answer play_arrow
question_answer 48) For traffic moving at 60 km/h along a circular track of radius 0.1 km, the correct angle of banking is:
A)
\[{{\tan }^{-1}}\left( \frac{{{60}^{2}}}{0.1} \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{{{(50/2)}^{2}}}{100\times 9.8} \right)\]
done
clear
C)
\[{{\tan }^{-1}}{{(60\times 0.1\times 9.8)}^{{1}/{2}\;}}\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{100\times 9.8}{{{(50/3)}^{3}}} \right)\]
done
clear
View Answer play_arrow
question_answer 49) Two projectiles are fired at different angles with the same magnitude of velocity, such that they have the same range. At what angles they might have been projected?
A)
\[25{}^\circ \text{ }and\text{ }65{}^\circ \]
done
clear
B)
\[35{}^\circ \text{ }and\text{ }75{}^\circ \]
done
clear
C)
\[10{}^\circ \text{ }and\text{ }50{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 50) If two non-zero vectors obey the relation \[\left| {\vec{P}} \right.+\vec{Q}\left| =\left| \vec{P}-\left. {\vec{Q}} \right| \right. \right.\] then the angle between the vectors \[\vec{P},\vec{Q}\] is :
A)
\[0{}^\circ \]
done
clear
B)
\[\pi \]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 51) A weightless spring which has a force constant k oscillates with a frequency n when a mass m is suspended from it. The spring is cut into two equal halves and a mass 2m is attached to one of the halves. The frequency of oscillation will not become:
A)
\[\sqrt{2n}\]
done
clear
B)
\[\frac{n}{\sqrt{2}}\]
done
clear
C)
\[\frac{2\pi }{\sqrt{b}}\]
done
clear
D)
n
done
clear
View Answer play_arrow
question_answer 52) A particle moves so that its acceleration a is given by a = - bx, where x is displacement from equilibrium position and b is a non-negative real constant. The time period of oscillation of the particle is :
A)
\[2\pi \sqrt{b}\]
done
clear
B)
\[\frac{2\pi }{b}\]
done
clear
C)
\[\frac{2\pi }{\sqrt{b}}\]
done
clear
D)
\[2\sqrt{\frac{\pi }{b}}\]
done
clear
View Answer play_arrow
question_answer 53) A tuning fork of frequency 500 cycles/s is sounded on a resonance tube. The first and second resonance is obtained at 17 cm and 52 cm. The velocity of sound in m/s is :
A)
175
done
clear
B)
350
done
clear
C)
525
done
clear
D)
700
done
clear
View Answer play_arrow
question_answer 54) Which of the following characteristics does not change due to the damping of simple harmonic motion?
A)
Angular frequency
done
clear
B)
Time period
done
clear
C)
Initial phase
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 55) A hollow charged metal sphere has a radius r. If the potential difference between its surface and a point at a distance 3 r from the centre is V, then electrical intensity at distance 3 r from the centre is:
A)
\[\frac{V}{2r}\]
done
clear
B)
\[\frac{V}{3r}\]
done
clear
C)
\[\frac{V}{4r}\]
done
clear
D)
\[\frac{V}{6r}\]
done
clear
View Answer play_arrow
question_answer 56) Current is flowing with a current density \[J=480\text{ }A/c{{m}^{2}}\] in a copper wire. Assuming that each copper atom contributes one free electron and given that: Avogadro number \[=6.0\times {{10}^{23}}\] atoms/mol Density of copper \[=\text{ }9.0\text{ }g/c{{m}^{3}}\] Atomic weight of copper \[=\text{ }64\text{ }g/mol\] Electronic charge \[=1.6\times {{10}^{-19}}C\] The drift velocity of electrons is :
A)
1 mm/s
done
clear
B)
2 mm/s
done
clear
C)
0.5 mm/s
done
clear
D)
0.36 mm/s
done
clear
View Answer play_arrow
question_answer 57) A tap supplies water at \[20{}^\circ C\]. A man takes 1L of water per minute at \[35{}^\circ C\] from a geyser connected to the tap. The power of geyser is :
A)
1050 W
done
clear
B)
2100 W
done
clear
C)
1500 W
done
clear
D)
3000 W
done
clear
View Answer play_arrow
question_answer 58) A charge is fired through a magentic field. The force acting on the charge is maximum when the angle between the direction of motion of charge and the magnetic field is :
A)
zero
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 59) At a place the angle of dip is 30°. If the horizontal component of earths magnetic field is H, then the total field intensity is :
A)
\[\frac{H}{2}\]
done
clear
B)
\[\frac{2H}{\sqrt{3}}\]
done
clear
C)
\[H\sqrt{2}\]
done
clear
D)
\[H\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 60) A particle is vibrating in simple harmonic motion with an amplitude of 4 cm. At what displacement from the equilibrium position is its energy half potential and half-kinetic?
A)
\[2\sqrt{2}cm\]
done
clear
B)
\[\sqrt{2}cm\]
done
clear
C)
2 cm
done
clear
D)
1 cm
done
clear
View Answer play_arrow
question_answer 61) A tuning fork of frequency 480 Hz produces 10 beats/s, when sounded With a sonometer string. A slight increase in tension in the sonometer string produces fewer beats/s than before. What was the frequency of sonometer string?
A)
470 Hz
done
clear
B)
490 Hz
done
clear
C)
480 Hz
done
clear
D)
460 Hz
done
clear
View Answer play_arrow
question_answer 62) A hollow metal sphere of radius 5 cm is charged such that potential at its surface is 10 V. The potential at the centre of the sphere is:
A)
zero volt
done
clear
B)
10 V
done
clear
C)
same as at point 5 cm away from the surface
done
clear
D)
same as at point 10 cm away from the surface
done
clear
View Answer play_arrow
question_answer 63) An electric dipole consisting of two opposite chargcs of \[2\times {{10}^{-6}}C\] each, separated by a distance of 3 cm is placed in an electric field of \[2\times {{10}^{5}}N/C.\] The maximum torque on the dipole is :
A)
\[12\times {{10}^{-1}}Nm\]
done
clear
B)
\[12\times {{10}^{-3}}Nm\]
done
clear
C)
\[24\times {{10}^{-1}}Nm\]
done
clear
D)
\[24\times {{10}^{-3}}Nm\]
done
clear
View Answer play_arrow
question_answer 64) A given piece of wire of length I and radius r is having a resistance R. This wire is stretched \[\frac{r}{2}\]uniformly to a wire of radius What is the new resistance?
A)
4R
done
clear
B)
8R
done
clear
C)
16K
done
clear
D)
2R
done
clear
View Answer play_arrow
question_answer 65)
Two thin long parallel wires separated by a distance b are carrying current i ampere each. The magnitude of the force per unit length exerted by one wire on the other is :
A)
\[\frac{{{\mu }_{0}}}{2\pi }\frac{{{i}^{2}}}{b}\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{2\pi }\frac{{{i}^{2}}}{{{b}^{2}}}\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{2\pi }\frac{i}{b}\]
done
clear
D)
\[\frac{{{\mu }_{0}}}{2\pi }\frac{i}{{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 66) To increase the range of a voltmeter, we need to connect a suitable:
A)
high resistance in parallel
done
clear
B)
high resistance in series
done
clear
C)
low resistance in series
done
clear
D)
low resistance in parallel
done
clear
View Answer play_arrow
question_answer 67) Two wires made up of the same material are of equal length but their radii are in the ratio of 1:2. On stretching each of these two strings by fundamental frequencies is :
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 68) An object producing a pitch of 400Hz flies past a stationary person. The object was moving in a straight line with a velocity 200m/s. The velocity of sound is 300 m/s. What is the change in frequency noted by the person as the object flies past him?
A)
1440 Hz
done
clear
B)
240 Hz
done
clear
C)
1200 Hz
done
clear
D)
960 Hz
done
clear
View Answer play_arrow
question_answer 69)
Four capacitors each of 1\[\mu F\] are connected as shown. The equivalent capacitance between P and Q is:
A)
4\[\mu F\]
done
clear
B)
\[\frac{1}{4}\mu F\]
done
clear
C)
\[\frac{3}{4}\mu F\]
done
clear
D)
\[\frac{4}{3}\mu F\]
done
clear
View Answer play_arrow
question_answer 70) The magnitude of electric field at distance r from an infinitely thin rod having a linear charge density \[\lambda \]is (use Gausss law)
A)
\[E=\frac{\lambda }{2\pi {{\varepsilon }_{0}}r}\]
done
clear
B)
\[E=\frac{2\lambda }{2\pi {{\varepsilon }_{0}}r}\]
done
clear
C)
\[E=\frac{2\lambda }{4\pi {{\varepsilon }_{0}}r}\]
done
clear
D)
\[E=\frac{4\lambda }{\pi {{\varepsilon }_{0}}r}\]
done
clear
View Answer play_arrow
question_answer 71) A 200 W and a 100 W bulb, both meant for operation at 220 V are connected in series. When connected to a 220 V supply the power consumed by the combination is :
A)
33.3 W
done
clear
B)
66.7 W
done
clear
C)
300 W
done
clear
D)
100 W
done
clear
View Answer play_arrow
question_answer 72) The sensitivity of a moving coil galvanometer depends on:
A)
angle of deflection
done
clear
B)
earths magnetic field
done
clear
C)
torsional constant of the spring
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 73) Domain formation is the necessary feature of:
A)
diamagnetic substances
done
clear
B)
paramagnetic substances
done
clear
C)
ferromagnetic substances
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 74) In a charged capacitor the energy stored in :
A)
the positive charges
done
clear
B)
the negative charges
done
clear
C)
the field between the plates
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 75) For a given temperature difference which of the following pairs will generate maximum thermo-emf?
A)
lead-nickel
done
clear
B)
copper-iron
done
clear
C)
gold-silver
done
clear
D)
antimony-bismuth
done
clear
View Answer play_arrow
question_answer 76)
The electrons identified by quantum numbers:
(I) \[n=4,l=1\]
(II) \[n=4,l=0\]
(III) \[n=3,l=2\]
(IV) \[n=2,l=1\]
can be placed in order of increasing energy from the lowest to highest as:
A)
(IV) < (II) < (III) < (I)
done
clear
B)
(II) < (IV) < (I) < (I)
done
clear
C)
(I) < (III) < (II) < (IY)
done
clear
D)
(III) < (I) < (IV) < CH)
done
clear
View Answer play_arrow
question_answer 77) Ground state electronic configuration of nitrogen atom can be represented as:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 78) The first emission line in the electronic spectrum of hydrogen in the Balmer series appears at\[c{{m}^{-1}}\]:
A)
\[\frac{9R}{400}c{{m}^{-1}}\]
done
clear
B)
\[\frac{7R}{144}c{{m}^{-1}}\]
done
clear
C)
\[\frac{3R}{4}c{{m}^{-1}}\]
done
clear
D)
\[\frac{5R}{36}c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 79) \[KMn{{O}_{4}}\]reacts with oxalic acid according to the equation \[2MnO_{4}^{-}+5{{C}_{2}}O_{4}^{2-}+16{{H}^{+}}\xrightarrow[{}]{{}}2M{{n}^{2+}}\]\[+10C{{O}_{2}}+8{{H}_{2}}O\] Here, 20 mL of\[0.1\text{ }M\text{ }KMn{{O}_{4}}\]is equivalent to:
A)
\[20\,mL\,of0.5M\,{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
done
clear
B)
\[50\,mL\,of\,0.1\,M\,{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
done
clear
C)
\[50\,mL\,of\,0.01\,M\,{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
done
clear
D)
\[20\,mL\,of\,0.1\,M\,{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 80) Half-life of a sample is 160 days. After 800 days, 1 g of the sample shall be reduced to:
A)
\[\frac{1}{2}g\]
done
clear
B)
\[\frac{1}{5}g\]
done
clear
C)
\[\frac{1}{4}g\]
done
clear
D)
\[\frac{1}{32}g\]
done
clear
View Answer play_arrow
question_answer 81) Radio-carbon dating was discovered by:
A)
G. N. Lewis
done
clear
B)
J. Williard Gibbs
done
clear
C)
W.F. Libby
done
clear
D)
W. Nernst
done
clear
View Answer play_arrow
question_answer 82) An aqueous solution of 6.3 g oxalic acid dihydrate is made up to 250 mL. The volume of 0.1 N sodium hydroxide required to completely neutralise 10 mL of this solution is:
A)
40 mL
done
clear
B)
20 mL
done
clear
C)
10 mL
done
clear
D)
4mL
done
clear
View Answer play_arrow
question_answer 83) How will increase of pressure affect the equation? \[C(s)+{{H}_{2}}O(g)CO(g)+{{H}_{2}}(g)\]
A)
Shift in the forward direction
done
clear
B)
Shift in the reverse direction
done
clear
C)
Increase in the yield of hydrogen
done
clear
D)
No effect
done
clear
View Answer play_arrow
question_answer 84) For which of the following reactions,\[{{K}_{p}}={{K}_{c}}\]?
A)
\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\]
done
clear
B)
\[{{N}_{2}}+{{O}_{2}}2NO\]
done
clear
C)
\[PC{{l}_{5}}PC{{l}_{3}}+C{{l}_{2}}\]
done
clear
D)
\[2S{{o}_{3}}2S{{O}_{2}}+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) Which of the following salts is most soluble?
A)
\[B{{i}_{2}}{{S}_{3}}({{K}_{sp}}=1\times {{10}^{-17}})\]
done
clear
B)
\[MnS({{K}_{sp}}=7\times {{10}^{-16}})\]
done
clear
C)
\[CuS({{K}_{sp}}=8\times {{10}^{-37}})\]
done
clear
D)
\[A{{g}_{2}}S({{K}_{sp}}=6\times {{10}^{-51}})\]
done
clear
View Answer play_arrow
question_answer 86) Ostwald dilution law is applicable to:
A)
strong electrolytes only
done
clear
B)
weak electrolytes only
done
clear
C)
non-electrolytes only
done
clear
D)
strong as well as weak electrolytes
done
clear
View Answer play_arrow
question_answer 87) In the reaction\[2A+B\xrightarrow[{}]{{}}{{A}_{2}}B,\]if the concentration of A is doubled and that of B is halved, then the rate of reaction will:
A)
increase by 4 times
done
clear
B)
decrease by 2 times
done
clear
C)
increase by 2 times
done
clear
D)
remains the same
done
clear
View Answer play_arrow
question_answer 88) The Arrhenius equation expressing the effect of temperature on the rate constant of a reaction is:
A)
\[k={{e}^{-E/RT}}\]
done
clear
B)
\[k=In\frac{E}{RT}\]
done
clear
C)
\[k=\frac{A.E}{RT}\]
done
clear
D)
\[k=A.{{e}^{-Ea/RT}}\]
done
clear
View Answer play_arrow
question_answer 89) If the half-time for a particular reaction is found to be constant and independent of the initial concentration of the reactants, then the reaction is of:
A)
first order
done
clear
B)
zero order
done
clear
C)
second order
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 90) Normality of\[2\text{ }M\text{ }{{H}_{2}}S{{O}_{4}}\]is:
A)
\[2N\]
done
clear
B)
\[4N\]
done
clear
C)
\[\frac{N}{2}\]
done
clear
D)
\[\frac{N}{4}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following is a non-colligative property?
A)
Elevation in boiling point
done
clear
B)
Osmotic pressure
done
clear
C)
Optical activity
done
clear
D)
Depression in freezing point
done
clear
View Answer play_arrow
question_answer 92) The freezing point of equivocal aqueous solution will be highest for:
A)
\[{{C}_{6}}{{H}_{5}}N{{H}_{3}}Cl\]
done
clear
B)
\[La{{(N{{O}_{3}})}_{3}}\]
done
clear
C)
glucose
done
clear
D)
\[Ca{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 93) The vant Hoff factor for \[0.1\,M\,Ba{{(N{{O}_{3}})}_{2}}\]solution is 2.74. The degree of dissociation is:
A)
91.3%
done
clear
B)
87%
done
clear
C)
100%
done
clear
D)
74%
done
clear
View Answer play_arrow
question_answer 94) The enthalpy of vaporisation of liquid water using the data: \[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(l);\]\[\Delta H=-285.77\text{ }kJ/mol\] \[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(g);\]\[\Delta H=-241.84\text{ }kJ/mol\]is:
A)
\[+\,43.93kJ/mol\]
done
clear
B)
\[-\,43.93\text{ }kJ/mol\]
done
clear
C)
\[527.61\text{ }kJ/mol\]
done
clear
D)
\[-\,527.61\text{ }kJ/mol\]
done
clear
View Answer play_arrow
question_answer 95) The enthalpy charge for the transition of liquid water to steam is\[40.8\text{ }kJ\text{ }mo{{l}^{-1}}\]at 373 K. Calculate the entropy of vaporisation of water.
A)
\[109.4\text{ }J{{K}^{-1}}\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[-109.4\text{ }J{{K}^{-1}}\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[218.8\text{ }J{{K}^{-1}}\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[-218.8\text{ }J{{K}^{-1}}\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) The occurrence of the reaction is impossible if:
A)
\[\Delta H\]is + ve;\[\Delta S\]is also + ve
done
clear
B)
\[\Delta H\]is\[-ve\];\[\Delta S\]is, also\[-ve\]
done
clear
C)
\[\Delta H\]is\[-ve\];\[\Delta S\]is +ve
done
clear
D)
\[\Delta H\]is + ve;\[\Delta S\]is\[-ve\]
done
clear
View Answer play_arrow
question_answer 97) The bond energy of an\[OH\]bond is 109 kcal/mol. When a mole of water is formed, then:
A)
109 kcals is released
done
clear
B)
218 kcals is absorbed
done
clear
C)
109 kcals is absorbed
done
clear
D)
218 kcals is released
done
clear
View Answer play_arrow
question_answer 98) Of the following reaction, only one is a redox reaction. Identify this reaction.
A)
\[Ca{{(OH)}_{2}}+2HCl\xrightarrow{{}}CaCl+2{{H}_{2}}O\]
done
clear
B)
\[2{{S}_{2}}O_{7}^{2-}+2{{H}_{2}}O\xrightarrow{{}}2SO_{4}^{2-}+4{{H}^{+}}\]
done
clear
C)
\[BaC{{l}_{2}}+MgS{{O}_{4}}\xrightarrow{{}}BaS{{O}_{4}}+MgC{{l}_{2}}\]
done
clear
D)
\[C{{u}_{2}}S+2FeO\xrightarrow{{}}2Cu+2Fe+S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) Oxidation number of nitrogen is highest in:
A)
\[{{N}_{3}}H\]
done
clear
B)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 100) Correct order of first ionization potential among the following elements\[Be,B,C,N,O\] is:
A)
\[B<Be<C<\text{O}<N\]
done
clear
B)
\[B<Be<C<N<O\]
done
clear
C)
\[Be<B<C<N<O\]
done
clear
D)
\[Be<B<C<O<N\]
done
clear
View Answer play_arrow
question_answer 101) The unit of equivalent conductivity is:
A)
ohm cm
done
clear
B)
\[oh{{m}^{-1}}c{{m}^{2}}\text{ }g\text{ }equi{{v}^{-1}}\]
done
clear
C)
\[ohm\text{ }c{{m}^{2}}\text{ }g\text{ }equiv.\]
done
clear
D)
\[oh{{m}^{-2}}c{{m}^{-2}}g\text{ }equiv.\]
done
clear
View Answer play_arrow
question_answer 102) In which of the crystals of ionic compounds would you expect maximum distance between the centres of cations and anions?
A)
\[LiF\]
done
clear
B)
\[CsF\]
done
clear
C)
\[CsI\]
done
clear
D)
\[Lil\]
done
clear
View Answer play_arrow
question_answer 103) 50 ml, of hydrogen diffuses through small hole from a vessel in 20 min time. Time taken for 40 mL of oxygen to diffuse out under similar conditions will be:
A)
12 min
done
clear
B)
32 min
done
clear
C)
8 min
done
clear
D)
64 min
done
clear
View Answer play_arrow
question_answer 104) A gas will approach ideal behaviour at:
A)
low temperature and high pressure
done
clear
B)
low temperature and low pressure
done
clear
C)
high temperature and low pressure
done
clear
D)
high temperature and high pressure
done
clear
View Answer play_arrow
question_answer 105) A colloidal system in which gas bubbles are dispersed in a liquid is known as:
A)
foam
done
clear
B)
aerosol
done
clear
C)
sol
done
clear
D)
emulsion
done
clear
View Answer play_arrow
question_answer 106) In which of the following, Tyndall effect is not observed?
A)
Smoke
done
clear
B)
Emulsions
done
clear
C)
Sugar solution
done
clear
D)
Gold sol
done
clear
View Answer play_arrow
question_answer 107) Which is not correct regarding the adsorption of a gas on surface of a solid?
A)
Enthalpy and entropy change is negative
done
clear
B)
Adsorption is more for some specific substance
done
clear
C)
On increasing temperature, adsorption increases progressively
done
clear
D)
It is a reversible reaction
done
clear
View Answer play_arrow
question_answer 108) The ionic radii of iso-electronic species \[N_{3}^{-},{{O}^{2-}}\]and\[{{F}^{-}}\]are in the order:
A)
1.36, 1.40, 1.71
done
clear
B)
1.36, 1.71, 1.40
done
clear
C)
1.71, 1.40, 1.36
done
clear
D)
1.71, 1.36, 1.40
done
clear
View Answer play_arrow
question_answer 109) Which of the following element is most electropositive?
A)
\[Al\]
done
clear
B)
\[Mg\]
done
clear
C)
\[P\]
done
clear
D)
\[S\]
done
clear
View Answer play_arrow
question_answer 110) The compound in which carbon atom uses \[s{{p}^{3}}-\]hybrid orbitals for bond formation?
A)
\[HCOOH\]
done
clear
B)
\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 111) The only .molecule having dipole moment is:
A)
2,2-dimethylpropane
done
clear
B)
trans-2-pentene
done
clear
C)
tran5-3-hexene
done
clear
D)
2,2,3,3-tetramethylbutane
done
clear
View Answer play_arrow
question_answer 112) \[{{N}_{2}}\]and\[{{O}_{2}}\]are converted to\[N_{2}^{+}\]and\[O_{2}^{+}\] respectively. Which of the following is not correct?
A)
In\[N_{2}^{+},\]the\[N-N\]bond weakens
done
clear
B)
In\[O_{2}^{+},O-O\]bond order increases
done
clear
C)
In\[O_{2}^{+},\]pararnagnetism decreases
done
clear
D)
\[N_{2}^{+}\] becomes diamagnetic
done
clear
View Answer play_arrow
question_answer 113) The hybridisation of carbon atoms in\[CC\] single bond of\[HC\equiv CCH=C{{H}_{2}}\]is:
A)
\[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}-s{{p}^{2}}\]
done
clear
C)
\[sp-s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{3}}-sp\]
done
clear
View Answer play_arrow
question_answer 114) Chemical A is used for water softening to remove temporary hardness. A reacts with sodium carbonate to generate caustic soda. When\[C{{O}_{2}}\]is bubbled through A, it turns cloudy. What is A?
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[CaO\]
done
clear
C)
\[Ca{{(OH)}_{2}}\]
done
clear
D)
\[Ca{{(HC{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following pentafluorides cant be formed?
A)
\[P{{F}_{5}}\]
done
clear
B)
\[As{{F}_{5}}\]
done
clear
C)
\[Sb{{F}_{5}}\]
done
clear
D)
\[Bi{{F}_{5}}\]
done
clear
View Answer play_arrow
question_answer 116) The correct order of increasing oxidising power is:
A)
\[{{F}_{2}}<C{{l}_{2}}<{{I}_{2}}>B{{r}_{2}}\]
done
clear
B)
\[{{F}_{2}}<B{{r}_{2}}<C{{l}_{2}}<{{I}_{2}}\]
done
clear
C)
\[C{{l}_{2}}<B{{r}_{2}}<{{F}_{2}}<{{I}_{2}}\]
done
clear
D)
\[{{I}_{2}}<B{{r}_{2}}<C{{l}_{2}}<{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 117) Amongst\[TiF_{6}^{2-},CoF_{6}^{3-},C{{u}_{2}}C{{l}_{2}}\]and\[NiCl_{4}^{2-}\]at. no.\[Ti=22,\text{ }Co=27,\text{ }Cu=29,\text{ }Ni=28\]). The colorless species are:
A)
\[CoF_{6}^{3-}\]and \[NiCl_{4}^{2-}\]
done
clear
B)
\[TiF_{6}^{2-}\]and \[CoF_{6}^{3-}\]
done
clear
C)
\[C{{u}_{2}}C{{l}_{2}}\]and \[NiCl_{4}^{2-}\]
done
clear
D)
\[TiFl_{6}^{2-}\]and\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 118) The number of\[SS\]bonds in sulphur trioxide is:
A)
three
done
clear
B)
two
done
clear
C)
one
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 119) Choose the correct statement:
A)
Transition elements have low melting points
done
clear
B)
Transition elements do not have catalytic activity
done
clear
C)
Transition elements exhibit variable oxidation states
done
clear
D)
Transition elements show inert pair effect
done
clear
View Answer play_arrow
question_answer 120) Two of the constituents of German silver are:
A)
\[Ag+Cu\]
done
clear
B)
\[Ag+Zn\]
done
clear
C)
\[Cu+Zn\]
done
clear
D)
\[Cu+Sn\]
done
clear
View Answer play_arrow
question_answer 121) Aufbau principle does not give the correct arrangement of filling up of atomic orbitals in:
A)
Cu and Zn
done
clear
B)
Co and Zn
done
clear
C)
Mn and Cr
done
clear
D)
Cu and Cr
done
clear
View Answer play_arrow
question_answer 122) Which of the following compounds exhibit linkage isomerism?
A)
\[[Co{{(en)}_{3}}]C{{l}_{3}}\]
done
clear
B)
\[[Co{{(NH 3)}_{6}}][Cr{{(CN)}_{6}}]\]
done
clear
C)
\[[Co{{(en)}_{2}}N{{O}_{2}}Cl]Br\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 123) IUPAC name of\[[Pt{{(N{{H}_{3}})}_{3}}]Br(N{{O}_{2}})Cl]Cl\]:
A)
triamminechlorobromonitro platinum(IV) chloride
done
clear
B)
triamminebromonitrochloro platinum(IV) chloride
done
clear
C)
triamminebromochloronitro platinum(IV) chloride
done
clear
D)
triamminenitrochlorobromo platinum(IV) chloride
done
clear
View Answer play_arrow
question_answer 124) According to postulates of Werners theory for co-ordination compounds, which of the following is true?
A)
Primary valencies are ionizable
done
clear
B)
Secondary valencies are ionizable
done
clear
C)
Only primary valencies are non-ionizable
done
clear
D)
Primary and secondary valencies are non- ionizable
done
clear
View Answer play_arrow
question_answer 125) Ferrocene is an example of:
A)
sand-wiched complex
done
clear
B)
pi-bonded complex
done
clear
C)
a complex in which all the five carbon atoms of cyclopentadiene anion are bonded to the metal
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 126) Which form of iron is least ductile?
A)
Hard steel
done
clear
B)
Cast iron
done
clear
C)
Mild steel
done
clear
D)
Wrought steel
done
clear
View Answer play_arrow
question_answer 127) Which of the following is known as Lunar caustic when in the fused state?
A)
Silver nitrate
done
clear
B)
Silver sulphate
done
clear
C)
Silver chloride
done
clear
D)
Sodium sulphate
done
clear
View Answer play_arrow
question_answer 128) One of the following metals is obtained by leaching its ore with dilute cyanide solution. Identify it.
A)
Titanium
done
clear
B)
Vanadium
done
clear
C)
Silver
done
clear
D)
Zinc
done
clear
View Answer play_arrow
question_answer 129)
Which of the following species could be expected to exhibit aromatic character?
A)
I and IV
done
clear
B)
II and IV
done
clear
C)
I and III
done
clear
D)
II and III
done
clear
View Answer play_arrow
question_answer 130) The correct IUPAC name of the following compound is: \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C=\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}\]\[-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
A)
5,6-dimethyl-8-methyldec-6-ene
done
clear
B)
6-butyl-5-ethyl-3-methyloct-4-ene
done
clear
C)
5,6-diethyl-3-methyldec-4-ene
done
clear
D)
2,4,5-triethylnon-3-ene
done
clear
View Answer play_arrow
question_answer 131) The kind of delocalisation involving sigma bond orbitals is called:
A)
inductive effect
done
clear
B)
hyper conjugation effect
done
clear
C)
electromeric effect
done
clear
D)
mesomeric effect
done
clear
View Answer play_arrow
question_answer 132) Which of the following compounds react with \[HBr\]obeying Markownikoffs rule?
A)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 133) How many cyclic isomers of\[{{C}_{5}}{{H}_{10}}\]are possible?
A)
Four
done
clear
B)
Three
done
clear
C)
Two
done
clear
D)
Six
done
clear
View Answer play_arrow
question_answer 134) The (R)and(S) enantiomers of an optically active compound differ in:
A)
their reactivity
done
clear
B)
their optical rotation of plane polarized light
done
clear
C)
their melting point
done
clear
D)
their solubility in achiral reagents
done
clear
View Answer play_arrow
question_answer 135) Which of the following. organic compounds exhibit acidic character?
A)
\[{{H}_{3}}C-C\equiv CH\]
done
clear
B)
\[{{H}_{3}}C-C\equiv C-C{{H}_{3}}\]
done
clear
C)
\[{{H}_{2}}C=C{{H}_{2}}\]
done
clear
D)
\[{{H}_{3}}C-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 136) Methane is produced by the hydrolysis of:
A)
\[A{{l}_{4}}{{C}_{3}}\]
done
clear
B)
\[Ca{{C}_{2}}\]
done
clear
C)
dry ice
done
clear
D)
\[n-{{C}_{3}}{{H}_{7}}MgBr\]
done
clear
View Answer play_arrow
question_answer 137) In the reaction of phenol with chloroform and aqueous solution of\[NaOH\]at\[{{70}^{o}}C,\]the electrophile attacking the ring is:
A)
\[CHC{{l}_{3}}\]
done
clear
B)
\[CHC{{l}_{2}}\]
done
clear
C)
\[{}_{\bullet }^{\bullet }CC{{l}_{2}}\]
done
clear
D)
\[COC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 138) Which of the following compounds can exist in optically active form?
A)
1-butanol
done
clear
B)
2-butanol
done
clear
C)
3-pentanol
done
clear
D)
4-heptanol
done
clear
View Answer play_arrow
question_answer 139) Chloroform is slowly oxidised by air in the presence of light and air to form:
A)
formyl chloride
done
clear
B)
trichloro methanol
done
clear
C)
phosgene
done
clear
D)
formaldehyde
done
clear
View Answer play_arrow
question_answer 140) Chlorination of toluene in the presence of light and heat followed by treatment with aqueous\[NaOH\]solution gives:
A)
o-cresol
done
clear
B)
p-cresol
done
clear
C)
benzoic acid
done
clear
D)
2,4-dihydroxytoluene
done
clear
View Answer play_arrow
question_answer 141) Formation of cyanohydrin from the reaction of acetone with HCN is called:
A)
electrophilic addition
done
clear
B)
nucleophilic addition
done
clear
C)
electrophilic substitution
done
clear
D)
nucleophilic substitution
done
clear
View Answer play_arrow
question_answer 142) In the following reaction,\[C{{H}_{3}}COCl\xrightarrow[Pd/{{H}_{2}}]{BaS{{O}_{4}}}X\]Identify X out of the following:
A)
acetaldehyde
done
clear
B)
propionaldehyde
done
clear
C)
acetone
done
clear
D)
acetic anhydride
done
clear
View Answer play_arrow
question_answer 143) Reaction of r-butylbromide with sodium methoxide produces:
A)
isobutene
done
clear
B)
isobutylene
done
clear
C)
sodium-t-butoxide
done
clear
D)
t-butylmethylether
done
clear
View Answer play_arrow
question_answer 144) The acid which does not contain\[COOH\] group is:
A)
ethanoicacid
done
clear
B)
picric acid
done
clear
C)
lactic acid
done
clear
D)
plamitic acid
done
clear
View Answer play_arrow
question_answer 145) The decreasing order of basic character of the three amines and ammonia is:
A)
\[N{{H}_{3}}>C{{h}_{3}}N{{H}_{2}}>{{C}_{2}}{{H}_{5}}N{{H}_{2}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}>C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}>{{C}_{2}}{{H}_{5}}N{{H}_{2}}>C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}>{{C}_{2}}{{H}_{5}}N{{H}_{2}}>N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 146) Aniline is treated with a mixture of sodium nitrite and hypophosphorus acid, the product formed is:
A)
aniline diazonium hypophosphate
done
clear
B)
benzene
done
clear
C)
anilinium hypophosphite
done
clear
D)
aniline diazonium hypophos-phite
done
clear
View Answer play_arrow
question_answer 147) Nitrosoamines\[({{R}_{2}}N-N=O)\]are soluble in water. On heating them with cone.\[{{H}_{2}}S{{O}_{4}},\]they give secondary amines. The reaction is called:
A)
Perkins reaction
done
clear
B)
Fries reaction
done
clear
C)
Libermann nitroso reaction
done
clear
D)
Etard reaction
done
clear
View Answer play_arrow
question_answer 148) The compound obtained by heating a mixture of\[{{1}^{o}}\]amine and chloroform with ethanolic potassium hydroxide is:
A)
an alkyl isocyanide
done
clear
B)
an alkyl isothiocyanate
done
clear
C)
an amide
done
clear
D)
an amide and nitro compound
done
clear
View Answer play_arrow
question_answer 149) Which of the following is not an amino-acid?
A)
Glycine
done
clear
B)
Alanine
done
clear
C)
Histidine
done
clear
D)
Benzidine
done
clear
View Answer play_arrow
question_answer 150) Which of the following is a fat soluble vitamin?
A)
Vitamin A
done
clear
B)
Riboflavin
done
clear
C)
Pyridoxin
done
clear
D)
Thiamine
done
clear
View Answer play_arrow
question_answer 151) Which of the following properties is shown by cytokinins?
A)
Delay leaf senescence
done
clear
B)
Promote stomatal closing
done
clear
C)
Cause leaf abscission
done
clear
D)
Promote seed dormancy
done
clear
View Answer play_arrow
question_answer 152) Read the statements given below. Which of these is wrong?
A)
Sporangiospores borne in the sporangium of Rhizopus are diploid structures
done
clear
B)
Rhizopus belongs to the class zygomycetes
done
clear
C)
Dominant phase in the like cycle of Chlamydomonas is haploid
done
clear
D)
Zoospores of Chlamydomonas are haploid
done
clear
View Answer play_arrow
question_answer 153) Development of gametophyte from sporophyte without formation of spores is called :
A)
apogamy
done
clear
B)
apospory
done
clear
C)
autogamy
done
clear
D)
hologamy
done
clear
View Answer play_arrow
question_answer 154) Read the statements given below Prothallus of Dryopteris is a ............. It bean archegonia on the ................... side. The blank positions are :
A)
sporophyte, dorsal
done
clear
B)
sporophyte, vental
done
clear
C)
gametophyte, dorsal
done
clear
D)
gametophyte, ventral
done
clear
View Answer play_arrow
question_answer 155) Which of the following characteristics does not occur in Pinus?
A)
The number of needles in a spur of Pinus roxburghii is three
done
clear
B)
Each vascular bundle in the long shoot of Pinus consists of xylem facing towards the centre of the shoot
done
clear
C)
Microsporophyll of Pinus bears two microsporangia
done
clear
D)
Pinus is a homosporous gymnosperm
done
clear
View Answer play_arrow
question_answer 156)
Pair the flower structures given in series-1 to their equivalent in series-11. Series-I Series-II A. Pollen grains 1. Microsporangia B. Pollen sacs 2. Microspores C. Stamens 3. Microsporophylls
The correct pairing is:
A)
A-1 B-2 C-3
done
clear
B)
A-2 B-1 C-3
done
clear
C)
A-3 B-1 C-2
done
clear
D)
A-1 B-3 C-2
done
clear
View Answer play_arrow
question_answer 157) In a plant the peduncle is elongated and it bears pedicillate flowers. The older flowers lie towards the base and the younger ones near the apex. The growth of the peduncle continues and more flowers are added. The inflorescence is :
A)
raceme
done
clear
B)
corymb
done
clear
C)
umbel
done
clear
D)
head
done
clear
View Answer play_arrow
question_answer 158) Which of these cells is the largest cell of the ovule?
A)
Antipodal cell
done
clear
B)
Central cell
done
clear
C)
Megaspore mother cell
done
clear
D)
The size of the cells varies from species to species and none of the given above can be treated as largest
done
clear
View Answer play_arrow
question_answer 159) Vegetative fertilization leading to the formation of endosperm refers to :
A)
fusion of male gamete with diploid secondary nucleus
done
clear
B)
fusion of female gamete with diploid secondary nucleus
done
clear
C)
fusion of two diploid vegetative cells
done
clear
D)
fusion of two male gametes
done
clear
View Answer play_arrow
question_answer 160)
Assign the seeds to their respective food reserve categories : Seed Category A. Maize 1. Endospermic B. Mustard 2. Nonendospermic C. Pea
The correct pairing is:
A)
A-1 B-1 C-2
done
clear
B)
A-1 B-2 C-2
done
clear
C)
A-2 B-2 C-1
done
clear
D)
A-1 B-2 C-1
done
clear
View Answer play_arrow
question_answer 161) Murashige and Skoogs medium is used for :
A)
isolation of fungal strains
done
clear
B)
culture of bacteria
done
clear
C)
raising plants through micro propagation
done
clear
D)
culture of protein rich cyanobacterium Spirulina
done
clear
View Answer play_arrow
question_answer 162) Which of the following plants does not have Rhizobium containing root nodules?
A)
Phaseolus
done
clear
B)
Pinus
done
clear
C)
Pisum
done
clear
D)
Cicer
done
clear
View Answer play_arrow
question_answer 163) Which of the following pesticides is a herbicide?
A)
2, 4-D
done
clear
B)
Malathion
done
clear
C)
Lindane
done
clear
D)
BHC
done
clear
View Answer play_arrow
question_answer 164) Allethrin is a commonly used :
A)
fertilizer
done
clear
B)
herbicide
done
clear
C)
growth hormone
done
clear
D)
insecticide
done
clear
View Answer play_arrow
question_answer 165) Urea is used in the soil to supplement:
A)
phosphorus
done
clear
B)
nitrogen
done
clear
C)
potassium
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 166) The first ever experimental evidence showing DNA as genetic material in bacteriophages came from the studies of:
A)
Beadle and Tatum
done
clear
B)
Weismann
done
clear
C)
Hershey and Chase
done
clear
D)
Schleiden and Schwann
done
clear
View Answer play_arrow
question_answer 167) Anti-codon is a base triplet on :
A)
m-RNA complementary to base sequence on r-RNA
done
clear
B)
m-RNA complementary to base sequence on t-RNA
done
clear
C)
t-RNA complementary to base sequence on r-RNA
done
clear
D)
r-RNA complementary to base sequence on m-RNA
done
clear
View Answer play_arrow
question_answer 168) Which one of the following nitrogenous bases is not present in RNA?
A)
Thymine
done
clear
B)
Uracil
done
clear
C)
Guanine
done
clear
D)
Cytosine
done
clear
View Answer play_arrow
question_answer 169) A person with XXY sex chromosomes suffers from:
A)
Downs syndrome
done
clear
B)
Klinefelters syndrome
done
clear
C)
Turners syndrome
done
clear
D)
AIDS
done
clear
View Answer play_arrow
question_answer 170) A condition characterized by not having an exact number of chromosomes in a-multiple of haploid set is called:
A)
polyploidy
done
clear
B)
synploidy
done
clear
C)
aneuploidy
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 171) Phenomenon of crossing over in diploid organisms is responsible for:
A)
linkages between genes
done
clear
B)
recombination between linked genes
done
clear
C)
segregation between genes
done
clear
D)
dominance of gene
done
clear
View Answer play_arrow
question_answer 172) Which of the following base sequence acts as a terminal codon during protein synthesis?
A)
AUG
done
clear
B)
GCG
done
clear
C)
UAG
done
clear
D)
AGA
done
clear
View Answer play_arrow
question_answer 173) Activation of an amino acid during protein synthesis requiers a participation of specific molecule of:
A)
m-RNA
done
clear
B)
t-RNA
done
clear
C)
r-RNA
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 174) An extra chromosomal DNA which can be used as vector in gene cloning is called :
A)
transposon
done
clear
B)
intron
done
clear
C)
exon
done
clear
D)
plasmid
done
clear
View Answer play_arrow
question_answer 175) Largest gland present in human body is :
A)
salivary gland
done
clear
B)
parotid gland
done
clear
C)
pituitary
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 176) A doctor advises a patient to include yellow fruits, carrots and butter in his diet. What deficiency disease do you think the patient is suffering from ?
A)
Night blindness
done
clear
B)
Colour blindness
done
clear
C)
Kwashiorkor disease
done
clear
D)
Marasmus disease
done
clear
View Answer play_arrow
question_answer 177) Volume of air breathed in and out while at rest is called :
A)
residual volume
done
clear
B)
tidal volume
done
clear
C)
vital volume
done
clear
D)
total lung capacity
done
clear
View Answer play_arrow
question_answer 178) What is the principal cation in human blood?
A)
Potassium
done
clear
B)
Sodium
done
clear
C)
Calcium
done
clear
D)
Manganese
done
clear
View Answer play_arrow
question_answer 179) Endocrine gland are :
A)
ductless glands whose secretions pour directly into blood
done
clear
B)
have ducts and pour their secretions into blood directly
done
clear
C)
have ducts which straightway pour secretions into target organs
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 180) Heart beat originates in :
A)
Purkinjes fibers
done
clear
B)
SA node
done
clear
C)
AV node
done
clear
D)
Chordae tendinae
done
clear
View Answer play_arrow
question_answer 181) Structural and functional unit of contractile apparatus in striated muscle is called :
A)
cross bridge
done
clear
B)
my of fibril
done
clear
C)
Z-band
done
clear
D)
sarcomere
done
clear
View Answer play_arrow
question_answer 182) Pulmonary artery differs from pulmonary vein in having :
A)
no endothelium
done
clear
B)
strong valves
done
clear
C)
Brunners cells
done
clear
D)
thick muscular walls
done
clear
View Answer play_arrow
question_answer 183) MSH is produced by :
A)
thyroid
done
clear
B)
anterior pituitary
done
clear
C)
posterior pituitary
done
clear
D)
pars inter medial is
done
clear
View Answer play_arrow
question_answer 184) What is the space between arachnoid and pia mater ?
A)
Supra arachnoid space
done
clear
B)
Sub arachnoid space
done
clear
C)
Subdural space
done
clear
D)
Meninges
done
clear
View Answer play_arrow
question_answer 185) Cerebellum portion of brain is :
A)
concerned with the maintenance of posture/equilibrium
done
clear
B)
responsibile for olfactory functions
done
clear
C)
controls optic functions
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 186) Which of the following hormones does not play any role in menstruation?
A)
GH
done
clear
B)
FSH
done
clear
C)
LH
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 187) Nephridia of earthworm are performing same functions as :
A)
gills of prawn
done
clear
B)
flame cells of Planaria
done
clear
C)
trachea of insects
done
clear
D)
nematoblasts of Hydra
done
clear
View Answer play_arrow
question_answer 188) Hollow bones are characteristic to:
A)
reptiles
done
clear
B)
birds
done
clear
C)
mammals
done
clear
D)
fishes
done
clear
View Answer play_arrow
question_answer 189) Body cavity o(Hydra is called :
A)
haemocoel
done
clear
B)
coelenterons
done
clear
C)
enterocoel
done
clear
D)
pseudocoel
done
clear
View Answer play_arrow
question_answer 190) Clitellum of earthworm is comprised of following body segments :
A)
14, 15, 16
done
clear
B)
13,14, 15
done
clear
C)
15, 16, 17
done
clear
D)
10,11,12
done
clear
View Answer play_arrow
question_answer 191) To which taxonomic group does a whale belong to?
A)
Fishes
done
clear
B)
Reptiles
done
clear
C)
Arthropoda
done
clear
D)
Mammals
done
clear
View Answer play_arrow
question_answer 192) Salamander belongs to :
A)
reptiles
done
clear
B)
amphibians
done
clear
C)
echinoderms
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 193) Which of the following statements is correct?
A)
Organs which are different in basic structure and origins but have similar functions are called analogous organs
done
clear
B)
Organs which are different in basic structure and origin but have dissimilar functions are called analogous organs
done
clear
C)
Organs which are similar in basic structure and origin but have different functions are called analogous organs
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 194) Who proposed the Theory of origin of species by natural selection ?
A)
August Weismann
done
clear
B)
de Vries
done
clear
C)
Charles Darwin
done
clear
D)
Charles Darwin and Alfred Wallace
done
clear
View Answer play_arrow
question_answer 195) One of the following factors does not play any role in understanding the concept of evolution. Which one is this factor?
A)
Genetic variation
done
clear
B)
Natural selection
done
clear
C)
Isolation
done
clear
D)
Embryogenesis
done
clear
View Answer play_arrow
question_answer 196) Which of the following statements is correct?
A)
Australopithecus is the real ancestor of man
done
clear
B)
Homo rectus is the ancestor of man
done
clear
C)
Neanderthal man is the direct ancestor of Homo sapiens
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 197) Which of the following diseases is not caused by virus?
A)
Mumps
done
clear
B)
Rabies
done
clear
C)
AIDS
done
clear
D)
Tuberculosis
done
clear
View Answer play_arrow
question_answer 198) Amniocentesis is a technique used to :
A)
determine errors in amino acid metabolism in embryo
done
clear
B)
pin point specific cardiac ailments in embryo
done
clear
C)
determine any hereditary genetic abnormality in embryo
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 199) Caffeine is a stimulant present in :
A)
coffee
done
clear
B)
tea
done
clear
C)
cold drinks
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 200) Which one of the following diseases is communicable?
A)
Rickets
done
clear
B)
Amoebiasis
done
clear
C)
Diabetes
done
clear
D)
Cancer
done
clear
View Answer play_arrow
question_answer 201) CAD stands for :
A)
Carotid Arterial Dysfunction
done
clear
B)
Cerebral Artery Dysfunction
done
clear
C)
Coronary Artery Disease
done
clear
D)
Calcium Activated Disease
done
clear
View Answer play_arrow
question_answer 202) What is correct for a person with blood group O ?
A)
The person has no antigen for both A and B antibodies
done
clear
B)
His blood has antibodies for both A and B antigens
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 203) What is true for DDT? It is :
A)
Not a pollutant
done
clear
B)
a non-degradable pollutant
done
clear
C)
an antibiotic
done
clear
D)
an antiseptic agent
done
clear
View Answer play_arrow
question_answer 204) Which of the following statements is wrong?
A)
Sucrose is a disaccharide
done
clear
B)
Cellulose is a polysaccharide
done
clear
C)
Glycine is a sulphur containing amino acid
done
clear
D)
Uracil is a pyrimidine
done
clear
View Answer play_arrow
question_answer 205) Each phospholipid molecule in a cell membrane consists of:
A)
one polar head and two non-polar tail
done
clear
B)
one polar head and one polar tail
done
clear
C)
one non-polar head and one polar tail
done
clear
D)
one non-polar head and one polar tail
done
clear
View Answer play_arrow
question_answer 206) Smooth endoplasmic reticulum acts as a major site for synthesis of:
A)
lipids and steroids
done
clear
B)
proteins
done
clear
C)
ribosomes
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 207) Which of the following statements is correct?
A)
Mitochondria contain circular DNA, but chloroplasts lack it
done
clear
B)
Chloroplasts contain circular DNA, but mitochondria lack it
done
clear
C)
Neither mitochondria nor chloroplasts contain any DNA
done
clear
D)
Both mitochondria and chloroplasts contain circular DNA
done
clear
View Answer play_arrow
question_answer 208)
Read the statements given below with regard to the functions performed by Golgi apparatus? A. Transport and chemically modify the materials contained within it B. Secrete mucin in the respiratory tract C. Secrete slime in the insectivorous plants which of the following is the correct answer?
A)
A is wrong but B and C correct
done
clear
B)
B is wrong but A and C correct
done
clear
C)
B and C wrong but A is correct
done
clear
D)
All A, B and C are correct
done
clear
View Answer play_arrow
question_answer 209) Which of the following cellular organelles is/are bound by a single membrane? peroxisomes, lysosomes, mitochondria
A)
Only peroxisomes but not lysosomes and mitochondria
done
clear
B)
Both peroxisomes and lysosomes but not mitochondria
done
clear
C)
All of the three organelles
done
clear
D)
None of the three organelles
done
clear
View Answer play_arrow
question_answer 210) Which of the following is not present in cell vacuoles ?
A)
Hydrolytic enzymes
done
clear
B)
Latex of the rubber plant
done
clear
C)
DNA
done
clear
D)
Anthocyanins of the flowers
done
clear
View Answer play_arrow
question_answer 211) Acrosome is a type of:
A)
lysosome
done
clear
B)
flagellum
done
clear
C)
ribosome
done
clear
D)
basal body
done
clear
View Answer play_arrow
question_answer 212)
Match the series-I and II for organismic respiration. Series-I Series II A. Respiration in bacteria 1. Mitochondria B. Respiration in cyanobacteria 2. Cytoplasmic membrane C. Respiration in eukaryotic cells 3. Mesosomes
The correct pairing is :
A)
A-2 B-3 C-1
done
clear
B)
A-3 B-2 C-1
done
clear
C)
A-1 B-3 C-2
done
clear
D)
A-2 B-1 C-3
done
clear
View Answer play_arrow
question_answer 213) Which of the following plants has haustorial roots?
A)
Pea
done
clear
B)
Trapa
done
clear
C)
Lily
done
clear
D)
Cuscuta
done
clear
View Answer play_arrow
question_answer 214)
Match the following series : Series-I Series-II A. Opuntia 1. Stem thorns B. Asparagus 2. Phylloclades C. Citrus 3. Cladodes
The correct pairing is :
A)
A-1 B-2 C-3
done
clear
B)
A-2 B-3 C-1
done
clear
C)
A-3 B-2 C-1
done
clear
D)
A-2 B-1 C-3
done
clear
View Answer play_arrow
question_answer 215)
Read the statements A and B given below : A. In a vessel septa among the adjoining cells get dissolved B. Companion cells are a component of phloem The right answer is :
A)
Both statements are wrong
done
clear
B)
A is wrong but B is correct
done
clear
C)
B is wrong but A is correct
done
clear
D)
Both A and B are correct
done
clear
View Answer play_arrow
question_answer 216) Vascular bundles in which xylem and phloem occur as separate bundles are known as :
A)
collateral
done
clear
B)
bicollateral
done
clear
C)
radial
done
clear
D)
amphivasal
done
clear
View Answer play_arrow
question_answer 217) Cork cambium of dicot originates from :
A)
epiblema
done
clear
B)
pericycle
done
clear
C)
cambium of vascular bundles
done
clear
D)
endodermis
done
clear
View Answer play_arrow
question_answer 218) Vascular bundles of monocot stem are generally:
A)
conjoint, collateral and closed
done
clear
B)
conjoint, collateral and open
done
clear
C)
conjoint, concentric and closed
done
clear
D)
conjoint, bicollateral and open
done
clear
View Answer play_arrow
question_answer 219)
Pair the species with the type of wood : Species Type of wood A. Tectona grandis 1. Soft wood B. Cedrus deodara 2. Hard wood D. Shorea robusta C. Dalbergia sisso
The correct pairing is :
A)
A-1 B-2 C-2 D-1
done
clear
B)
1 1 2 2 A-1 B-1 C-2 D-2
done
clear
C)
A-2 B-1 C-2 D-2
done
clear
D)
A-2 B-1 C-1 D-2
done
clear
View Answer play_arrow
question_answer 220) Which of the following has maximum water potential?
A)
Pure water
done
clear
B)
2% sucrose solution
done
clear
C)
4% glucose solution
done
clear
D)
10% sodium chloride solution
done
clear
View Answer play_arrow
question_answer 221) Which of the following cations accumulate in the guard cells in light to cause stomatal opening?
A)
\[C{{a}^{2+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[{{K}^{+}}\]
done
clear
D)
\[M{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 222) Which of the following acts as an acceptor of \[C{{O}_{2}}\] in \[{{C}_{3}}\] plants ?
A)
Glycerate phosphate
done
clear
B)
Glucose disphosphate
done
clear
C)
Sedoheptulose
done
clear
D)
Ribulose bisphosphate
done
clear
View Answer play_arrow
question_answer 223)
Which of the following characteristics out of A, B and C arc exhibited by \[{{C}_{4}}\]plants? A. Kranz anatomy B.The product of photosynthesis is oxaloacetic acid C. Both PEP carboxylase and Ribulose- bisphosphate carboxylase act as carboxy- lating enzymes
The correct answer is:
A)
only A and B, but not C
done
clear
B)
only B and C, but not A
done
clear
C)
only A and C, but not B
done
clear
D)
all A, B and C
done
clear
View Answer play_arrow
question_answer 224) Glycolysis leads to the formation of 2 molecules of:
A)
oxaloacetic acid
done
clear
B)
pyruvic acid
done
clear
C)
citric acid
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 225) Inhibition of enzyme activity by a molecule, which reversibly modifies the structure of the active site of the enzyme is called :
A)
competitive inhibition
done
clear
B)
non-competitive reversible inhibition
done
clear
C)
allosteric inhibition
done
clear
D)
none of the above
done
clear
View Answer play_arrow
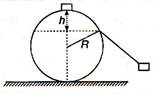
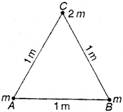
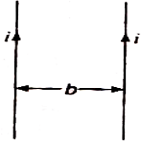
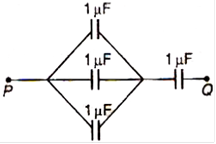
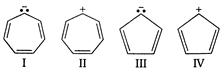 Select the correct answer from the following:
Select the correct answer from the following:
