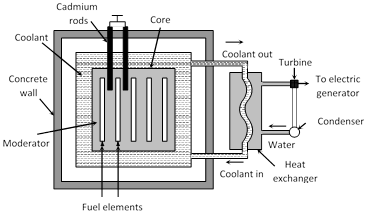
 (1) Fissionable material (Fuel) : The fissionable material used in the reactor is called the fuel of the reactor. Uranium isotope \[({{U}^{235}})\] Thorium isotope \[(T{{h}^{232}})\] and Plutonium isotopes \[(P{{u}^{239}},\,\,P{{u}^{240}}\,\,\text{and}\,\,P{{u}^{241}})\] are the most commonly used fuels in the reactor.
(2) Moderator : Moderator is used to slow down the fast moving neutrons. Most commonly used moderators are graphite and heavy water \[({{D}_{2}}O)\].
(3) Control Material : Control material is used to control the chain reaction and to maintain a stable rate of reaction. This material controls the number of neutrons available for the fission. For example, cadmium rods are inserted into the core of the reactor because they can absorb the neutrons. The neutrons available for fission are controlled by moving the cadmium rods in or out of the core of the reactor.
(4) Coolant : Coolant is a cooling material which removes the heat generated due to fission in the reactor. Commonly used coolants are water, \[C{{O}_{2}}\] nitrogen etc.
(5) Protective shield : A protective shield in the form a concrete thick wall surrounds the core of the reactor to save the persons working around the reactor from the hazardous radiations.
(6) Uses of nuclear reactor
(i) In electric power generation.
(ii) To produce radioactive isotopes for their use in medical science, agriculture and industry.
(iii) In manufacturing of \[P{{u}^{239}}\] which is used in atom bomb.
(iv) They are used to produce neutron beam of high intensity which is used in the treatment of cancer and nuclear research.
(1) Fissionable material (Fuel) : The fissionable material used in the reactor is called the fuel of the reactor. Uranium isotope \[({{U}^{235}})\] Thorium isotope \[(T{{h}^{232}})\] and Plutonium isotopes \[(P{{u}^{239}},\,\,P{{u}^{240}}\,\,\text{and}\,\,P{{u}^{241}})\] are the most commonly used fuels in the reactor.
(2) Moderator : Moderator is used to slow down the fast moving neutrons. Most commonly used moderators are graphite and heavy water \[({{D}_{2}}O)\].
(3) Control Material : Control material is used to control the chain reaction and to maintain a stable rate of reaction. This material controls the number of neutrons available for the fission. For example, cadmium rods are inserted into the core of the reactor because they can absorb the neutrons. The neutrons available for fission are controlled by moving the cadmium rods in or out of the core of the reactor.
(4) Coolant : Coolant is a cooling material which removes the heat generated due to fission in the reactor. Commonly used coolants are water, \[C{{O}_{2}}\] nitrogen etc.
(5) Protective shield : A protective shield in the form a concrete thick wall surrounds the core of the reactor to save the persons working around the reactor from the hazardous radiations.
(6) Uses of nuclear reactor
(i) In electric power generation.
(ii) To produce radioactive isotopes for their use in medical science, agriculture and industry.
(iii) In manufacturing of \[P{{u}^{239}}\] which is used in atom bomb.
(iv) They are used to produce neutron beam of high intensity which is used in the treatment of cancer and nuclear research. | Controlled chain reaction | Uncontrolled chain reaction |
| Controlled by artificial method | No control over this type more...
In nuclear fission, three neutrons are produced along with the release of large energy. Under favourable conditions, these neutrons can cause further fission of other nuclei, producing large number of neutrons. Thus a chain of nuclear fissions is established which continues until the whole of the uranium is consumed.
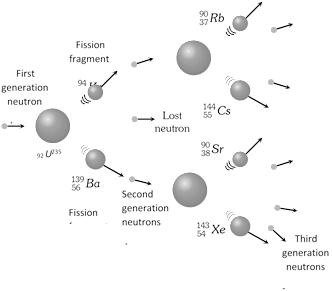 In the chain reaction, the number of nuclei undergoing fission increases very fast. So, the energy produced takes a tremendous magnitude very soon.
In the chain reaction, the number of nuclei undergoing fission increases very fast. So, the energy produced takes a tremendous magnitude very soon.
(1) The process of splitting of a heavy nucleus into two lighter nuclei of comparable masses (after bombardment with a energetic particle) with liberation of energy is called nuclear fission.
(2) The phenomenon of nuclear fission was discovered by scientist Ottohann and F. Strassman and was explained by N. Bohr and J.A. Wheeler on the basis of liquid drop model of nucleus.
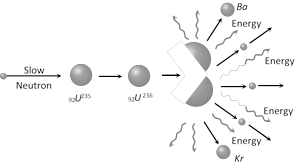
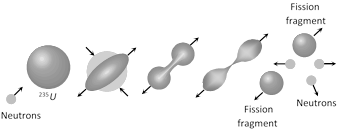 (3) Fission reaction of \[{{U}^{\mathbf{235}}}\]
\[_{92}{{U}^{235}}+{{\ }_{0}}{{n}^{1}}\to \ \underset{\text{(unstable nucleus)}}{\mathop{_{92}{{U}^{236}}}}\,\to {{\ }_{56}}B{{a}^{141}}+{{\ }_{36}}K{{r}^{92}}+{{3}_{0}}{{n}^{1}}+Q\]
(4) The energy released in \[{{U}^{\mathbf{235}}}\] fission is about 200 MeV or 0.8 MeV per nucleon.
(5) By fission of \[_{92}{{U}^{235}}\], on an average 2.5 neutrons are liberated. These neutrons are called fast neutrons and their energy is about 2 MeV (for each). These fast neutrons can escape from the reaction so as to proceed the chain reaction they are need to slow down.
(6) Fission of \[{{U}^{\mathbf{235}}}\] occurs by slow neutrons only (of energy about 1eV) or even by thermal neutrons (of energy about 0.025 eV).
(7) 50 kg of \[{{U}^{\mathbf{235}}}\] on fission will release \[\approx 4\times {{10}^{15}}J\] of energy. This is equivalence to 20,000 tones of TNT explosion. The nuclear bomb dropped at Hiroshima had this much explosion power.
(8) The mass of the compound nucleus must be greater than the sum of masses of fission products.
(9) The \[\frac{\text{Binding energy}}{\text{A}}\] of compound nucleus must be less than that of the fission products.
(10) It may be pointed out that it is not necessary that in each fission of uranium, the two fragments \[_{56}Ba\] and \[_{36}Kr\] are formed but they may be any stable isotopes of middle weight atoms.
(11) Same other \[{{U}^{\mathbf{235}}}\] fission reactions are
\[_{92}{{U}^{235}}+{{\,}_{0}}{{n}^{1}}\to {{\,}_{54}}X{{e}^{140}}+{{\,}_{38}}S{{r}^{94}}+\,{{2}_{0}}{{n}^{1}}\]
\[\to {{\,}_{57}}L{{a}^{148}}+{{\,}_{35}}B{{r}^{85}}+3{{\,}_{0}}{{n}^{1}}\]
\[\to \] Many more
(12) The neutrons released during the fission process are called prompt neutrons.
(13) Most of energy released appears in the form of kinetic energy of fission fragments.
(3) Fission reaction of \[{{U}^{\mathbf{235}}}\]
\[_{92}{{U}^{235}}+{{\ }_{0}}{{n}^{1}}\to \ \underset{\text{(unstable nucleus)}}{\mathop{_{92}{{U}^{236}}}}\,\to {{\ }_{56}}B{{a}^{141}}+{{\ }_{36}}K{{r}^{92}}+{{3}_{0}}{{n}^{1}}+Q\]
(4) The energy released in \[{{U}^{\mathbf{235}}}\] fission is about 200 MeV or 0.8 MeV per nucleon.
(5) By fission of \[_{92}{{U}^{235}}\], on an average 2.5 neutrons are liberated. These neutrons are called fast neutrons and their energy is about 2 MeV (for each). These fast neutrons can escape from the reaction so as to proceed the chain reaction they are need to slow down.
(6) Fission of \[{{U}^{\mathbf{235}}}\] occurs by slow neutrons only (of energy about 1eV) or even by thermal neutrons (of energy about 0.025 eV).
(7) 50 kg of \[{{U}^{\mathbf{235}}}\] on fission will release \[\approx 4\times {{10}^{15}}J\] of energy. This is equivalence to 20,000 tones of TNT explosion. The nuclear bomb dropped at Hiroshima had this much explosion power.
(8) The mass of the compound nucleus must be greater than the sum of masses of fission products.
(9) The \[\frac{\text{Binding energy}}{\text{A}}\] of compound nucleus must be less than that of the fission products.
(10) It may be pointed out that it is not necessary that in each fission of uranium, the two fragments \[_{56}Ba\] and \[_{36}Kr\] are formed but they may be any stable isotopes of middle weight atoms.
(11) Same other \[{{U}^{\mathbf{235}}}\] fission reactions are
\[_{92}{{U}^{235}}+{{\,}_{0}}{{n}^{1}}\to {{\,}_{54}}X{{e}^{140}}+{{\,}_{38}}S{{r}^{94}}+\,{{2}_{0}}{{n}^{1}}\]
\[\to {{\,}_{57}}L{{a}^{148}}+{{\,}_{35}}B{{r}^{85}}+3{{\,}_{0}}{{n}^{1}}\]
\[\to \] Many more
(12) The neutrons released during the fission process are called prompt neutrons.
(13) Most of energy released appears in the form of kinetic energy of fission fragments.
The process by which the identity of a nucleus is changed when it is bombarded by an energetic particle is called nuclear reaction. The general expression for the nuclear reaction is as follows.
\[\underset{(\text{Parent nucleus)}}{\mathop{X}}\,+\underset{(\text{Incident particle)}}{\mathop{a}}\,\xrightarrow{\,}\]
\[\underset{\text{(Compound nucleus)}}{\mathop{C}}\,\,\xrightarrow{\,}\underset{(\text{Compound nucleus)}}{\mathop{Y}}\,+\underset{\text{(Product particles)}}{\mathop{b}}\,+\underset{(\text{Energy)}}{\mathop{Q}}\,\]
Here X and a are known as reactants and Y and b are known as products. This reaction is known as (a, b) reaction and can be represented as X(a, b) Y
(1) Q value or energy of nuclear reaction : The energy absorbed or released during nuclear reaction is known as Q-value of nuclear reaction.
Q-value = (Mass of reactants ? mass of products)\[{{c}^{2}}\] Joules
= (Mass of reactants ? mass of products) amu
If \[Q<0,\] The nuclear reaction is known as endothermic. (The energy is absorbed in the reaction)
If \[Q>0,\] The nuclear reaction is known as exothermic (The energy is released in the reaction)
(2) Law of conservation in nuclear reactions
(i) Conservation of mass number and charge number : In the following nuclear reaction
\[_{2}H{{e}^{4}}+{{\,}_{7}}{{N}^{14}}\to {{\,}_{8}}{{O}^{17}}+{{\,}_{1}}{{H}^{1}}\]
Mass number (A)\[\to \] Before the reaction After the reaction
4 +14 = 18 17 + 1 = 18
Charge number (Z) \[\to \] 2 + 7 = 9 8 + 1 = 9
(ii) Conservation of momentum : Linear momentum/angular momentum of particles before the reaction is equal to the linear/angular momentum of the particles after the reaction. That is \[\Sigma p=0\]
(iii) Conservation of energy : Total energy before the reaction is equal to total energy after the reaction. Term Q is added to balance the total energy of the reaction.
(3) Common nuclear reactions : The nuclear reactions lead to artificial transmutation of nuclei. Rutherford was the first to carry out artificial transmutation of nitrogen to oxygen in the year 1919.
\[_{2}H{{e}^{4}}+{{\,}_{7}}{{N}^{14}}\to {{\,}_{9}}{{F}^{18}}\to {{\,}_{8}}{{O}^{17}}+{{\,}_{1}}{{H}^{1}}\]
It is called \[(\alpha ,\,\,p)\] reaction. Some other nuclear reactions are given as follows.
\[(p,\,n)\] reaction \[\Rightarrow \] \[_{1}{{H}^{1}}+{{\,}_{5}}{{B}^{11}}\to {{\,}_{6}}{{C}^{12}}\to {{\,}_{6}}{{C}^{11}}+{{\,}_{0}}{{n}^{1}}\]
\[(p,\,\,\alpha )\]reaction \[\Rightarrow \]\[_{1}{{H}^{1}}+{{\,}_{3}}L{{i}^{11}}\to {{\,}_{4}}B{{e}^{8}}\to {{\,}_{2}}H{{e}^{4}}+{{\,}_{2}}H{{e}^{4}}\]
\[(p,\,\,\gamma )\] reaction \[\Rightarrow \]\[_{1}{{H}^{1}}+{{\,}_{6}}{{C}^{12}}\to {{\,}_{7}}{{N}^{13}}\to {{\,}_{7}}{{N}^{13}}+\gamma \]
\[(n,\,\,p)\] reaction \[\Rightarrow \]\[_{0}{{n}^{1}}+{{\,}_{7}}{{N}^{14}}\to {{\,}_{7}}{{N}^{15}}\to {{\,}_{6}}{{C}^{14}}+{{\,}_{1}}{{H}^{1}}\]
\[(\gamma ,\,n)\] reaction \[\Rightarrow \]\[\gamma +{{\,}_{1}}{{H}^{2}}\to {{\,}_{1}}{{H}^{1}}\,+{{\,}_{0}}{{n}^{1}}\]
It is the graph between binding energy per nucleon and total number of nucleons (i.e. mass number A)
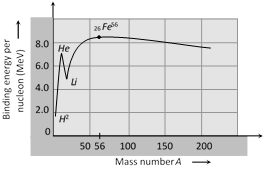 (1) Some nuclei with mass number \[A<20\] have large binding energy per nucleon than their neighbour nuclei. For example \[_{2}H{{e}^{4}},{{\,}_{4}}B{{e}^{8}},{{\,}_{6}}{{C}^{12}},{{\,}_{8}}{{O}^{16}}\text{ and}{{\text{ }}_{\text{10}}}N{{e}^{20}}\]. These nuclei are more stable than their neighbours.
(2) The binding energy per nucleon is maximum for nuclei of mass number \[A=56\] \[{{(}_{26}}F{{e}^{56}})\]. It's value is 8.8 MeV per nucleon.
(3) For nuclei having \[A>56,\] binding energy per nucleon gradually decreases for uranium \[(A=238),\] the value of binding energy per nucleon drops to 7.5 MeV.
(1) Some nuclei with mass number \[A<20\] have large binding energy per nucleon than their neighbour nuclei. For example \[_{2}H{{e}^{4}},{{\,}_{4}}B{{e}^{8}},{{\,}_{6}}{{C}^{12}},{{\,}_{8}}{{O}^{16}}\text{ and}{{\text{ }}_{\text{10}}}N{{e}^{20}}\]. These nuclei are more stable than their neighbours.
(2) The binding energy per nucleon is maximum for nuclei of mass number \[A=56\] \[{{(}_{26}}F{{e}^{56}})\]. It's value is 8.8 MeV per nucleon.
(3) For nuclei having \[A>56,\] binding energy per nucleon gradually decreases for uranium \[(A=238),\] the value of binding energy per nucleon drops to 7.5 MeV.
(1) Mass defect \[(\Delta m)\]: It is found that the mass of a nucleus is always less than the sum of masses of it's constituent nucleons in free state. This difference in masses is called mass defect. Hence mass defect
\[\Delta m=\] Sum of masses of nucleons - Mass of nucleus
\[=\left\{ Z{{m}_{p}}+(A-Z){{m}_{n}} \right\}-M=\left\{ Z{{m}_{p}}+Z{{m}_{e}}+(A-Z){{m}_{z}} \right\}-M'\]
where \[{{m}_{p}}=\] Mass of proton, \[{{m}_{n}}=\]Mass of each neutron, \[{{m}_{e}}=\]Mass of each electron
M = Mass of nucleus, Z = Atomic number, A = Mass number, M¢ = Mass of atom as a whole.
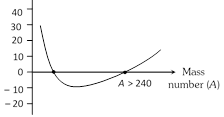
(2) Packing fraction : Mass defect per nucleon is called packing fraction
Packing fraction (f ) \[=\frac{\Delta m}{A}=\frac{M-A}{A}\] where M = Mass of nucleus, A = Mass number
Packing fraction measures the stability of a nucleus. Smaller the value of packing fraction, larger is the stability of the nucleus.
(i) Packing fraction may be of positive, negative or zero value.
(ii) At A = 16, f\[\to \] Zero
 (3) Binding energy (B.E.) : The neutrons and protons in a stable nucleus are held together by nuclear forces and energy is needed to pull them infinitely apart (or the same energy is released during the formation of the nucleus). This energy is called the binding energy of the nucleus.
or
The binding energy of a nucleus may be defined as the energy equivalent to the mass defect of the nucleus.
If \[\Delta m\] is mass defect then according to Einstein's mass energy relation
Binding energy \[=\Delta m\cdot {{c}^{2}}=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)\}-M]\cdot {{c}^{2}}\]
(This binding energy is expressed in joule, because \[\Delta m\]is measured in kg)
If \[\Delta m\] is measured in amu then binding energy \[=\Delta m\,\,amu\]\[=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)-M]amu=\Delta m\times 931\,\,MeV\]
(4) Binding energy per nucleon : The average energy required to release a nucleon from the nucleus is called binding energy per nucleon.
Binding energy per nucleon
\[=\frac{\text{Total binding energy}}{\begin{matrix} \text{Mass number (}i.e\text{. total number} \\ \text{ of nucleons)} \\ \end{matrix}}\]\[=\frac{\Delta m\times 931}{A}\frac{MeV}{Nucleon}\]
Binding energy per nucleon \[\propto \] Stability of nucleus
(3) Binding energy (B.E.) : The neutrons and protons in a stable nucleus are held together by nuclear forces and energy is needed to pull them infinitely apart (or the same energy is released during the formation of the nucleus). This energy is called the binding energy of the nucleus.
or
The binding energy of a nucleus may be defined as the energy equivalent to the mass defect of the nucleus.
If \[\Delta m\] is mass defect then according to Einstein's mass energy relation
Binding energy \[=\Delta m\cdot {{c}^{2}}=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)\}-M]\cdot {{c}^{2}}\]
(This binding energy is expressed in joule, because \[\Delta m\]is measured in kg)
If \[\Delta m\] is measured in amu then binding energy \[=\Delta m\,\,amu\]\[=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)-M]amu=\Delta m\times 931\,\,MeV\]
(4) Binding energy per nucleon : The average energy required to release a nucleon from the nucleus is called binding energy per nucleon.
Binding energy per nucleon
\[=\frac{\text{Total binding energy}}{\begin{matrix} \text{Mass number (}i.e\text{. total number} \\ \text{ of nucleons)} \\ \end{matrix}}\]\[=\frac{\Delta m\times 931}{A}\frac{MeV}{Nucleon}\]
Binding energy per nucleon \[\propto \] Stability of nucleus
Among about 1500 known nuclides, less than 260 are stable. The others are unstable that decay to form other nuclides by emitting \[\alpha ,\,\,\beta -\]particles and \[\gamma -\]EM waves. (This process is called radioactivity). The stability of nucleus is determined by many factors. Few such factors are given below :
(1) Neutron-proton ratio \[\left( \frac{N}{Z}\,\text{Ratio} \right)\] : The chemical properties of an atom are governed entirely by the number of protons (Z) in the nucleus, the stability of an atom appears to depend on both the number of protons and the number of neutrons.
(i) For lighter nuclei, the greatest stability is achieved when the number of protons and neutrons are approximately equal \[(N\approx Z)\] i.e. \[\frac{N}{Z}=1\]
(ii) Heavy nuclei are stable only when they have more neutrons than protons. Thus heavy nuclei are neutron rich compared to lighter nuclei (for heavy nuclei, more is the number of protons in the nucleus, greater is the electrical repulsive force between them. Therefore more neutrons are added to provide the strong attractive forces necessary to keep the nucleus stable.)
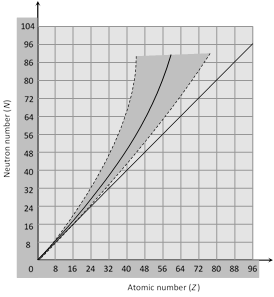 (iii) Figure shows a plot of N verses Z for the stable nuclei. For mass number upto about A = 40. For larger value of Z the nuclear force is unable to hold the nucleus together against the electrical repulsion of the protons unless the number of neutrons exceeds the number of protons. At Bi (Z = 83, A = 209), the neutron excess in \[N-Z=43\]. There are no stable nuclides with \[Z>83\].
(2) Even or odd numbers of Z or N : The stability of a nuclide is also determined by the consideration whether it contains an even or odd number of protons and neutrons.
(i) It is found that an even-even nucleus (even Z and even N) is more stable (60% of stable nuclide have even Z and even N).
(ii) An even-odd nucleus (even Z and odd N) or odd-even nuclide (odd Z and even N) is found to be lesser sable while the odd-odd nucleus is found to be less stable.
(iii) Only five stable odd-odd nuclides are known : \[_{1}{{H}^{2}},\,{{\,}_{3}}L{{i}^{6}},\,{{\,}_{5}}B{{e}^{10}},\,{{\,}_{7}}{{N}^{14}}\,\text{and}{{\text{ }}_{\text{75}}}T{{a}^{180}}\]
(3) Binding energy per nucleon : The stability of a nucleus is determined by value of it's binding energy per nucleon. In general higher the value of binding energy per nucleon, more stable the nucleus is
(iii) Figure shows a plot of N verses Z for the stable nuclei. For mass number upto about A = 40. For larger value of Z the nuclear force is unable to hold the nucleus together against the electrical repulsion of the protons unless the number of neutrons exceeds the number of protons. At Bi (Z = 83, A = 209), the neutron excess in \[N-Z=43\]. There are no stable nuclides with \[Z>83\].
(2) Even or odd numbers of Z or N : The stability of a nuclide is also determined by the consideration whether it contains an even or odd number of protons and neutrons.
(i) It is found that an even-even nucleus (even Z and even N) is more stable (60% of stable nuclide have even Z and even N).
(ii) An even-odd nucleus (even Z and odd N) or odd-even nuclide (odd Z and even N) is found to be lesser sable while the odd-odd nucleus is found to be less stable.
(iii) Only five stable odd-odd nuclides are known : \[_{1}{{H}^{2}},\,{{\,}_{3}}L{{i}^{6}},\,{{\,}_{5}}B{{e}^{10}},\,{{\,}_{7}}{{N}^{14}}\,\text{and}{{\text{ }}_{\text{75}}}T{{a}^{180}}\]
(3) Binding energy per nucleon : The stability of a nucleus is determined by value of it's binding energy per nucleon. In general higher the value of binding energy per nucleon, more stable the nucleus is
When an energetic g-ray photon falls on a heavy substance. It is absorbed by some nucleus of the substance and an electron and a positron are produced. This phenomenon is called pair production and may be represented by the following equation
\[\underset{(\gamma -\text{photon)}}{\mathop{h\nu }}\,\,\,\,\,\,\,\,\,=\,\,\,\,\,\,\,\underset{(\text{Positron)}}{\mathop{_{1}{{\beta }^{0}}}}\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\underset{(\text{Electron)}}{\mathop{_{-1}{{\beta }^{0}}}}\,\]
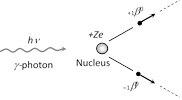 The rest-mass energy of each of positron and electron is
\[{{E}_{0}}={{m}_{0}}{{c}^{2}}=(9.1\times {{10}^{31}}kg)\times {{(3.0\times {{10}^{8}}m/s)}^{2}}\] \[=8.2\times {{10}^{14}}J=\mathbf{0}.\mathbf{51}MeV\]
Hence, for pair-production it is essential that the energy of g-photon must be at least \[2\times 0.51\text{ }=1.02MeV\]. If the energy of \[\gamma -\]photon is less than this, it would cause photo-electric effect or Compton effect on striking the matter.
The converse phenomenon pair-annihilation is also possible. Whenever an electron and a positron come very close to each other, they annihilate each other by combining together and two \[\gamma -\]photons (energy) are produced. This phenomenon is called pair annihilation and is represented by the following equation.
\[\underset{(\text{Positron)}}{\mathop{_{+1}{{\beta }^{0}}}}\,\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\underset{(\text{E}lectron\text{)}}{\mathop{_{-1}{{\beta }^{0}}}}\,\,\,\,\,\,\,\,=\,\,\,\,\,\,\,\underset{(\gamma \text{-photon})}{\mathop{h\nu }}\,\,\,\,\,+\,\,\,\underset{(\gamma \text{-photon})}{\mathop{h\nu }}\,\]
The rest-mass energy of each of positron and electron is
\[{{E}_{0}}={{m}_{0}}{{c}^{2}}=(9.1\times {{10}^{31}}kg)\times {{(3.0\times {{10}^{8}}m/s)}^{2}}\] \[=8.2\times {{10}^{14}}J=\mathbf{0}.\mathbf{51}MeV\]
Hence, for pair-production it is essential that the energy of g-photon must be at least \[2\times 0.51\text{ }=1.02MeV\]. If the energy of \[\gamma -\]photon is less than this, it would cause photo-electric effect or Compton effect on striking the matter.
The converse phenomenon pair-annihilation is also possible. Whenever an electron and a positron come very close to each other, they annihilate each other by combining together and two \[\gamma -\]photons (energy) are produced. This phenomenon is called pair annihilation and is represented by the following equation.
\[\underset{(\text{Positron)}}{\mathop{_{+1}{{\beta }^{0}}}}\,\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\underset{(\text{E}lectron\text{)}}{\mathop{_{-1}{{\beta }^{0}}}}\,\,\,\,\,\,\,\,=\,\,\,\,\,\,\,\underset{(\gamma \text{-photon})}{\mathop{h\nu }}\,\,\,\,\,+\,\,\,\underset{(\gamma \text{-photon})}{\mathop{h\nu }}\,\]
(1) In nuclear physics, a convenient unit of mass is the unified atomic mass unit abbreviated u.
(2) The amu is defined as \[\frac{1}{12}th\] mass of a \[_{B}{{C}^{12}}\] at on.
(3) 1 amu (or 1 u) =\[1.6605402\times {{10}^{27}}kg\].
(4) Masses of electron, proton and neutrons :
Mass of electron\[\text{(}{{m}_{e}})\text{ }=\text{ }9.1\times {{10}^{31}}kg=\text{ }0.0005486\,\,amu\],
Mass of proton \[\text{(}{{m}_{p}})=1.6726\times {{10}^{27}}kg=\text{ }1.007276\,\,amu\]
Mass of neutron \[\text{(}{{m}_{n}})=1.6750\times {{10}^{27}}kg=1.00865\,\,amu\],
Mass of hydrogen atom \[\text{(}{{m}_{e}}+{{m}_{p}}\text{)}=1.6729\times {{10}^{27}}kg=\text{ }1.0078\,\,amu\]
(5) The energy associated with a nuclear process is usually large, of the order of MeV.
(6) According to Einstein, mass and energy are inter convertible. The Einstein's mass energy relationship is given by \[E=m{{c}^{2}}\]
If \[m=1\,\,amu,\,\,c=3\times {{10}^{8}}m/sec\] then \[E=931\,MeV\] i.e. 1 amu is equivalent to 931 MeV or \[1\,\,amu\,\,(or\text{ }1u)=931MeV\]
\[(1\,u){{c}^{2}}=931MeV\]\[\Rightarrow \] \[1u=931\frac{MeV}{{{c}^{2}}}\] or \[{{c}^{2}}=931\frac{MeV}{u}\]
Neutral atomic masses for some light nuclides
|