 Peltier co-efficient \[\mathbf{(\pi )}\]: Heat absorbed or liberated at the junction is directly proportional to the charge passing through the junction i.e. \[H\propto Q\Rightarrow H=\pi Q;\] where \[\pi \] is called Peltier co-efficient. It's unit is J/C or volt.
Peltier co-efficient of a junction is the amount of heat absorbed or liberated per sec. When 1 amp of current is passed to the thermo couple.
It is found that \[\pi =T\frac{dE}{dT}=T\times S\]; where T is in Kelvin and \[\frac{dE}{dT}=P=\] Seebeck coefficient S
Peltier co-efficient \[\mathbf{(\pi )}\]: Heat absorbed or liberated at the junction is directly proportional to the charge passing through the junction i.e. \[H\propto Q\Rightarrow H=\pi Q;\] where \[\pi \] is called Peltier co-efficient. It's unit is J/C or volt.
Peltier co-efficient of a junction is the amount of heat absorbed or liberated per sec. When 1 amp of current is passed to the thermo couple.
It is found that \[\pi =T\frac{dE}{dT}=T\times S\]; where T is in Kelvin and \[\frac{dE}{dT}=P=\] Seebeck coefficient S 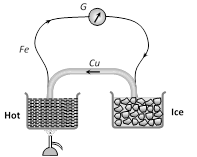 (2) Seebeck series : The magnitude and direction of thermo emf in a thermocouple depends not only on the temperature difference between the hot and cold junctions but also on the nature of metals constituting the thermo couple.
(i) Seebeck arranged different metals in the decreasing order of their electron density. Few metals forming the series are as below.
Sb, Fe, Cd, Zn, Ag, Au, Cr, Sn, Pb, Hg, Mn, Cu, Pt, Co, Ni, Bi
(ii) Thermo electric emf is directly proportional to the distance between the two metals in series. Farther the metals in the series forming the thermo couple greater is the thermo emf. Thus maximum thermo emf is obtained for Sb-Bi thermo couple.
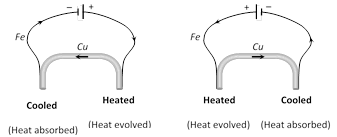
(iii) The current flow at the hot junction of the thermocouple is from the metal occurring later in the series towards that occurring earlier, Thus, in the copper-iron thermocouple the current flows from copper (Cu) to iron (Fe) at the hot junction. This may be remembered easily by the hot coffee.
(3) Variation of thermo emf with temperature : In a thermocouple as the temperature of the hot junction increases keeping the cold junction at constant temperature (say \[{{0}^{o}}C\]). The thermo emf increases till it becomes maximum at a certain temperature.
(2) Seebeck series : The magnitude and direction of thermo emf in a thermocouple depends not only on the temperature difference between the hot and cold junctions but also on the nature of metals constituting the thermo couple.
(i) Seebeck arranged different metals in the decreasing order of their electron density. Few metals forming the series are as below.
Sb, Fe, Cd, Zn, Ag, Au, Cr, Sn, Pb, Hg, Mn, Cu, Pt, Co, Ni, Bi
(ii) Thermo electric emf is directly proportional to the distance between the two metals in series. Farther the metals in the series forming the thermo couple greater is the thermo emf. Thus maximum thermo emf is obtained for Sb-Bi thermo couple.
(iii) The current flow at the hot junction of the thermocouple is from the metal occurring later in the series towards that occurring earlier, Thus, in the copper-iron thermocouple the current flows from copper (Cu) to iron (Fe) at the hot junction. This may be remembered easily by the hot coffee.
(3) Variation of thermo emf with temperature : In a thermocouple as the temperature of the hot junction increases keeping the cold junction at constant temperature (say \[{{0}^{o}}C\]). The thermo emf increases till it becomes maximum at a certain temperature.
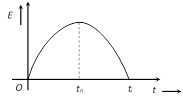 (i) Thermo electric emf is given by the equation \[E=\alpha \,t+\frac{1}{2}\beta \,{{t}^{2}}\] where \[\alpha \] and \[\beta \] are thermo electric constant having units are volt/\[^{o}C\] and volt/\[^{o}{{C}^{2}}\] respectively (t = temperature of hot junction). For E to be maximum (at \[t={{t}_{n}}\])
\[\frac{dE}{dt}=0\] i.e.\[\alpha +\beta \,{{t}_{n}}=0\Rightarrow {{t}_{n}}=-\frac{\alpha }{\beta }\]
(ii) The temperature of hot junction at which thermo emf becomes maximum is called neutral temperature \[({{t}_{n}})\]. Neutral temperature is constant for a thermo couple (e.g. for \[Cu-Fe,\,\,{{t}_{n}}={{270}^{o}}C\])
(iii) Neutral temperature is independent of the temperature of cold junction.
(iv) If temperature of hot junction increases beyond neutral temperature, thermo emf start decreasing and at a particular temperature it becomes zero, on heating slightly further, the direction of emf is reversed. This temperature of hot junction is called temperature of inversion \[({{t}_{i}})\].
(v) Relation between \[{{t}_{n}},\,{{t}_{i}}\] and \[{{t}_{c}}\] is \[{{t}_{n}}=\frac{{{t}_{i}}+{{t}_{c}}}{2}\]
(4) Thermo electric power : The rate of change of thermo emf with the change in the temperature of the hot junction is called thermoelectric power.
It is also given by the slope of parabolic curve representing the variation of thermo emf with temperature of the hot junction, as discussed in previous section.
The thermo electric power \[\left( \frac{dE}{dt} \right)\] is also called Seebeck coefficient. Differentiating both sides of the equation of thermo emf with respect to t, we have thermoelectric power \[P=\frac{dE}{dt}=\frac{d}{dt}(\alpha more...
(i) Thermo electric emf is given by the equation \[E=\alpha \,t+\frac{1}{2}\beta \,{{t}^{2}}\] where \[\alpha \] and \[\beta \] are thermo electric constant having units are volt/\[^{o}C\] and volt/\[^{o}{{C}^{2}}\] respectively (t = temperature of hot junction). For E to be maximum (at \[t={{t}_{n}}\])
\[\frac{dE}{dt}=0\] i.e.\[\alpha +\beta \,{{t}_{n}}=0\Rightarrow {{t}_{n}}=-\frac{\alpha }{\beta }\]
(ii) The temperature of hot junction at which thermo emf becomes maximum is called neutral temperature \[({{t}_{n}})\]. Neutral temperature is constant for a thermo couple (e.g. for \[Cu-Fe,\,\,{{t}_{n}}={{270}^{o}}C\])
(iii) Neutral temperature is independent of the temperature of cold junction.
(iv) If temperature of hot junction increases beyond neutral temperature, thermo emf start decreasing and at a particular temperature it becomes zero, on heating slightly further, the direction of emf is reversed. This temperature of hot junction is called temperature of inversion \[({{t}_{i}})\].
(v) Relation between \[{{t}_{n}},\,{{t}_{i}}\] and \[{{t}_{c}}\] is \[{{t}_{n}}=\frac{{{t}_{i}}+{{t}_{c}}}{2}\]
(4) Thermo electric power : The rate of change of thermo emf with the change in the temperature of the hot junction is called thermoelectric power.
It is also given by the slope of parabolic curve representing the variation of thermo emf with temperature of the hot junction, as discussed in previous section.
The thermo electric power \[\left( \frac{dE}{dt} \right)\] is also called Seebeck coefficient. Differentiating both sides of the equation of thermo emf with respect to t, we have thermoelectric power \[P=\frac{dE}{dt}=\frac{d}{dt}(\alpha more... 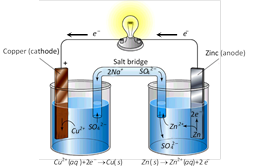 It is an arrangement in which the chemical energy is converted into electrical energy due to chemical action taking place in it.
(1) Primary cell : Is that cell in which electrical energy is produced due to chemical energy. In the primary cell, chemical reaction is irreversible. This cell can not be recharged. Examples of primary cells are Voltaic cell, Daniel cell, Leclanche cell and Dry cell etc.
(2) Secondary cell : A secondary cell is that cell in which the electrical energy is first stored up as a chemical energy and when the current is taken from the cell, the chemical energy is reconverted into electrical energy. In the secondary cell chemical reactions are reversible. The secondary cells are also called storage cell or accumulator. The commonly used secondary cells is lead accumulator.
(3) Defects In a primary cell : In voltaic cell there are two main defects arises.
It is an arrangement in which the chemical energy is converted into electrical energy due to chemical action taking place in it.
(1) Primary cell : Is that cell in which electrical energy is produced due to chemical energy. In the primary cell, chemical reaction is irreversible. This cell can not be recharged. Examples of primary cells are Voltaic cell, Daniel cell, Leclanche cell and Dry cell etc.
(2) Secondary cell : A secondary cell is that cell in which the electrical energy is first stored up as a chemical energy and when the current is taken from the cell, the chemical energy is reconverted into electrical energy. In the secondary cell chemical reactions are reversible. The secondary cells are also called storage cell or accumulator. The commonly used secondary cells is lead accumulator.
(3) Defects In a primary cell : In voltaic cell there are two main defects arises.
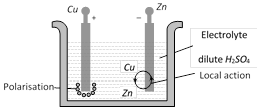 Local action : It arises due to the presence of impurities of iron, carbon etc. on the surface of commercial Zn rod used as an electrode. The particles of these impurities and Zn in contact with sulphuric acid form minute voltaic cell in which small local electric currents are set up resulting in the wastage of Zn even when the cell is not sending the external current.
Removal : By amalgamating Zn rod with mercury (i.e. the surface of Zn is coated with Hg).
Polarisation : It arises, when the positive \[{{H}_{2}}\] ions, which are formed by the action of Zn on sulphuric acid, travel towards the Cu rod and after transferring, the positive charge converted into H2 gas atoms and get deposited in the form of neutral layer of a gas on the surface of Cu rod. This weakens the action of cell.
Removal : Either by brushing the anode the remove the layer or by using a depolariser (i.e. some oxidising agent \[Mn{{O}_{2}},\,CuS{{O}_{4}}\] etc which may oxidise \[{{H}_{2}}\] into water).
Thermo electric effect of current
Local action : It arises due to the presence of impurities of iron, carbon etc. on the surface of commercial Zn rod used as an electrode. The particles of these impurities and Zn in contact with sulphuric acid form minute voltaic cell in which small local electric currents are set up resulting in the wastage of Zn even when the cell is not sending the external current.
Removal : By amalgamating Zn rod with mercury (i.e. the surface of Zn is coated with Hg).
Polarisation : It arises, when the positive \[{{H}_{2}}\] ions, which are formed by the action of Zn on sulphuric acid, travel towards the Cu rod and after transferring, the positive charge converted into H2 gas atoms and get deposited in the form of neutral layer of a gas on the surface of Cu rod. This weakens the action of cell.
Removal : Either by brushing the anode the remove the layer or by using a depolariser (i.e. some oxidising agent \[Mn{{O}_{2}},\,CuS{{O}_{4}}\] etc which may oxidise \[{{H}_{2}}\] into water).
Thermo electric effect of current
 If two wires of different metals are joined at their ends so as to form two junctions, then the resulting arrangement is called a "Thermo couple".
If two wires of different metals are joined at their ends so as to form two junctions, then the resulting arrangement is called a "Thermo couple". | Element | Atomic weight | Atomic number | Valency | E.C.E. (Z) in kg / C | |||||||
| Hydrogen | 1.0008 | 1 | 1 | \[10.4\times {{10}^{9}}\] | |||||||
| Oxygen | 15.999 | 8 | 2 | \[82.9\times {{10}^{9}}\] | |||||||
| Aluminium | 26.982 | more...
Current can produce or speed up chemical change, this ability of current is called chemical effect (shown by dc not by ac).
(1) Electrolytes : The liquids which allows the current to pass through them and also dissociates into ions on passing current through them are called electrolytes e.g. solutions of salts, acids and bases in water, etc.
Those liquids which do not allow current to pass through them are called insulators (e.g. vegetable oils, distilled water etc.)
Solutions of cane sugar, glycerin, alcohol etc. are examples of non-electrolytes.
(2) Electrolysis : The process of decomposition of electrolyte solution into ions on passing the current through it is called electrolysis.
Practical applications of electrolysis are Electrotyping, extraction of metals from the ores, Purification of metals, Manufacture of chemicals, Production of \[{{O}_{2}}\] and \[{{H}_{2}}\], Medical applications and electroplating.
(3) Electroplating : It is a process of depositing a thin layer of one metal over another metal by the method of electrolysis. The articles of cheap metals are coated with precious metals like silver and gold to make their look more attractive. The article to be electroplated is made the cathode and the metal to be deposited is made the anode. A soluble salt of the precious metal is taken as the electrolyte. (If gold is to be coated then auric chloride is used as electrolyte).
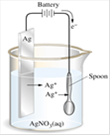 (4) Voltameter : The vessel in which the electrolysis is carried out is called a voltameter. It contains two electrodes and electrolyte. It is also known as electrolytic cell.
Types of voltameters
(4) Voltameter : The vessel in which the electrolysis is carried out is called a voltameter. It contains two electrodes and electrolyte. It is also known as electrolytic cell.
Types of voltameters
|