| \[n=\infty \] | Infinite | Infinite | \[0\,eV\] | |||||||||||||||
| \[n=4\] | Fourth | Third | \[-0.85\,eV\] | |||||||||||||||
| \[n=3\] |
| Quantity | Formula | Dependency on n and Z |
| (1) Angular speed | \[{{\omega }_{n}}=\frac{{{v}_{n}}}{{{r}_{n}}}=\frac{\pi m{{z}^{2}}{{e}^{4}}}{2\varepsilon _{0}^{2}{{n}^{3}}{{h}^{3}}}\] | \[{{\omega }_{n}}\propto \frac{{{Z}^{2}}}{{{n}^{3}}}\] |
| (2) Frequency | \[{{\nu }_{n}}=\frac{{{\omega }_{n}}}{2\pi }=\frac{m{{z}^{2}}{{e}^{4}}}{4\varepsilon _{0}^{2}{{n}^{3}}{{h}^{3}}}\] | \[{{\nu }_{n}}\propto \frac{{{Z}^{2}}}{{{n}^{3}}}\] |
| (3) Time period | \[{{T}_{n}}=\frac{1}{{{\nu }_{n}}}=\frac{4\varepsilon _{0}^{2}{{n}^{3}}{{h}^{3}}}{m{{z}^{2}}{{e}^{4}}}\] | \[{{T}_{n}}\propto \frac{{{n}^{3}}}{{{Z}^{2}}}\] |
| (4) Angular momentum | \[{{L}_{n}}=m{{v}_{n}}{{r}_{n}}=n\,\left( \frac{h}{2\pi } \right)\] | \[{{L}_{n}}\propto n\] |
| (5) Corresponding current | \[{{i}_{n}}=e{{\nu more...
(1) It is valid only for one electron atoms, e.g. : \[H,\,H{{e}^{+}},\,L{{i}^{+2}},\,N{{a}^{+1}}\] etc.
(2) Orbits were taken as circular but according to Sommerfield these are elliptical.
(3) Intensity of spectral lines could not be explained.
(4) Nucleus was taken as stationary but it also rotates on its own axis.
(5) It could not be explained the minute structure in spectrum line.
(6) This does not explain the Zeeman effect (splitting up of spectral lines in magnetic field) and Stark effect (splitting up in electric field)
(7) This does not explain the doublets in the spectrum of some of the atoms like sodium \[(5890\,\overset{\text{o}}{\mathop{\text{A}}}\,\,\And 5896\,\overset{\text{o}}{\mathop{\text{A}}}\,)\]
Bohr proposed a model for hydrogen atom which is also applicable for some lighter atoms in which a single electron revolves around a stationary nucleus of positive charge Ze (called hydrogen like atom)
Bohr's model is based on the following postulates.
(1) He postulated that an electron in an atom can move around the nucleus in certain circular stable orbits without emitting radiations.
(2) Bohr found that the magnitude of the electron's
Angular momentum is quantized i.e. \[L=m{{v}_{n}}{{r}_{n}}=n\,\left( \frac{h}{2\pi } \right)\]
where n = 1, 2, 3, ..... each value of n corresponds to a permitted value of the orbit radius.
\[{{r}_{n}}=\] Radius of \[{{n}^{th}}\] orbit, \[{{v}_{n}}=\] corresponding speed
(3) The radiation of energy occurs only when an electron jumps from one permitted orbit to another.
When electron jumps from higher energy orbit \[({{E}_{2}})\] to lower energy orbit \[({{E}_{1}})\] then difference of energies of these orbits i.e. \[{{E}_{2}}-{{E}_{1}}\] emits in the form of photon. But if electron goes from \[{{E}_{1}}\] to \[{{E}_{2}}\] it absorbs the same amount of energy.
(1) Stability of atom : It could not explain stability of atom because according to classical electrodynamics theory an accelerated charged particle should continuously radiate energy. Thus an electron moving in an circular path around the nucleus should also radiate energy and thus move into smaller and smaller orbits of gradually decreasing radius and it should ultimately fall into nucleus.
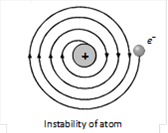 (2) According to this model the spectrum of atom must be continuous where as practically it is a line spectrum.
(3) It did not explain the distribution of electrons outside the nucleus.
(2) According to this model the spectrum of atom must be continuous where as practically it is a line spectrum.
(3) It did not explain the distribution of electrons outside the nucleus.
After \[\alpha -\]particles scattering experiment, following conclusions were made by Rutherford as regard as atomic structure :
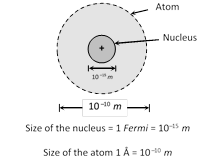 (1) Most of the mass (at least 99.95%) and all of the charge of an atom concentrated in a very small region is called atomic nucleus.
(2) Nucleus is positively charged and it's size is of the order of \[{{10}^{-15}}\,m\approx 1\] Fermi. The nucleus occupies only about \[{{10}^{-12}}\] of the total volume of the atom or less.
(3) In an atom there is maximum empty space and the electrons revolve around the nucleus in the same way as the planets revolve around the sun.
(1) Most of the mass (at least 99.95%) and all of the charge of an atom concentrated in a very small region is called atomic nucleus.
(2) Nucleus is positively charged and it's size is of the order of \[{{10}^{-15}}\,m\approx 1\] Fermi. The nucleus occupies only about \[{{10}^{-12}}\] of the total volume of the atom or less.
(3) In an atom there is maximum empty space and the electrons revolve around the nucleus in the same way as the planets revolve around the sun.
'Geiger and Marsden (students of Rutherford) studied the scattering of \[\alpha -\]particles by gold foil on the advice of Rutherford and made the following observations.
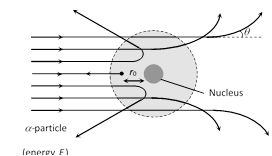 (1) Most of the \[\alpha -\]particles pass through the foil straight away undeflected.
(2) Some of them are deflected through small angles.
(3) A few \[\alpha -\]particles (1 in 1000) are deflected through the angle more than \[{{90}^{o}}\].
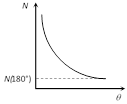
(4) A few \[\alpha -\]particles (very few) returned back i.e. deflected by \[{{180}^{o}}\].
(5) Number of scattered particles : \[N\propto \frac{1}{{{\sin }^{4}}(\theta /2)}\]
(1) Most of the \[\alpha -\]particles pass through the foil straight away undeflected.
(2) Some of them are deflected through small angles.
(3) A few \[\alpha -\]particles (1 in 1000) are deflected through the angle more than \[{{90}^{o}}\].
(4) A few \[\alpha -\]particles (very few) returned back i.e. deflected by \[{{180}^{o}}\].
(5) Number of scattered particles : \[N\propto \frac{1}{{{\sin }^{4}}(\theta /2)}\]
 (6) If t is the thickness of the foil and N is the number of \[\alpha -\]particles scattered in a particular direction (i.e. \[\theta =\]constant), it was observed that \[\frac{N}{t}=\text{constant}\]\[\Rightarrow \]\[\frac{{{N}_{1}}}{{{N}_{2}}}=\frac{{{t}_{1}}}{{{t}_{2}}}\]
(7) Distance of closest approach (Nuclear dimension) : The minimum distance from the nucleus up to which the \[\alpha -\]particle approach, is called the distance of closest approach \[({{r}_{0}})\]. At this distance the entire initial kinetic energy has been converted into potential energy so \[\frac{1}{2}m{{v}^{2}}=\frac{1}{4\pi {{\varepsilon }_{0}}}.\,\frac{(Ze)\,2e}{{{r}_{0}}}\]\[\Rightarrow \]\[{{r}_{0}}=\frac{Z{{e}^{2}}}{m{{v}^{2}}\pi {{\varepsilon }_{0}}}\]\[=\frac{4kZ{{e}^{2}}}{m{{v}^{2}}}\]
(8) Impact parameter (b) : The perpendicular distance of the velocity vector \[(\vec{\upsilon })\] of the \[\alpha -\]particle from the centre of the nucleus when it is far away from the nucleus is known as impact parameter. It is given as
(6) If t is the thickness of the foil and N is the number of \[\alpha -\]particles scattered in a particular direction (i.e. \[\theta =\]constant), it was observed that \[\frac{N}{t}=\text{constant}\]\[\Rightarrow \]\[\frac{{{N}_{1}}}{{{N}_{2}}}=\frac{{{t}_{1}}}{{{t}_{2}}}\]
(7) Distance of closest approach (Nuclear dimension) : The minimum distance from the nucleus up to which the \[\alpha -\]particle approach, is called the distance of closest approach \[({{r}_{0}})\]. At this distance the entire initial kinetic energy has been converted into potential energy so \[\frac{1}{2}m{{v}^{2}}=\frac{1}{4\pi {{\varepsilon }_{0}}}.\,\frac{(Ze)\,2e}{{{r}_{0}}}\]\[\Rightarrow \]\[{{r}_{0}}=\frac{Z{{e}^{2}}}{m{{v}^{2}}\pi {{\varepsilon }_{0}}}\]\[=\frac{4kZ{{e}^{2}}}{m{{v}^{2}}}\]
(8) Impact parameter (b) : The perpendicular distance of the velocity vector \[(\vec{\upsilon })\] of the \[\alpha -\]particle from the centre of the nucleus when it is far away from the nucleus is known as impact parameter. It is given as
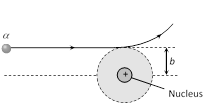 \[b=\frac{Z{{e}^{2}}\cot (\theta /2)}{4\pi {{\varepsilon }_{0}}\left( \frac{1}{2}m{{v}^{2}} \right)}\,\]\[\Rightarrow \]\[b\propto \cot (\theta /2)\]
For large b, \[\alpha \]particles will go undeviated and for small b the \[\alpha -\]particle will suffer large scattering.
\[b=\frac{Z{{e}^{2}}\cot (\theta /2)}{4\pi {{\varepsilon }_{0}}\left( \frac{1}{2}m{{v}^{2}} \right)}\,\]\[\Rightarrow \]\[b\propto \cot (\theta /2)\]
For large b, \[\alpha \]particles will go undeviated and for small b the \[\alpha -\]particle will suffer large scattering.
J.J. Thomson gave the first idea regarding structure of atom. According to this model.
(1) An atom is a solid sphere in which entire and positive charge and it's mass is uniformly distributed and in which negative charge (i.e. electron) are embedded like seeds in watermelon.
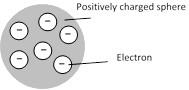 (2) This model explained successfully the phenomenon of thermionic emission, photoelectric emission and ionization.
(3) The model fail to explain the scattering of \[\alpha -\]particles and it cannot explain the origin of spectral lines observed in the spectrum of hydrogen and other atoms.
(2) This model explained successfully the phenomenon of thermionic emission, photoelectric emission and ionization.
(3) The model fail to explain the scattering of \[\alpha -\]particles and it cannot explain the origin of spectral lines observed in the spectrum of hydrogen and other atoms.
(i) In study of crystal structure : Structure of DNA was also determined using X-ray diffraction.
(ii) In medical science
(iii) In radiography
(iv) In radio therapy
(v) In engineering
(vi) In laboratories
(vii) In detective department
(viii) In art the change occurring in old oil paintings can be examined by X-rays.
Mosley studied the characteristic X-ray spectrum of a number of a heavy elements and concluded that the spectra of different elements are very similar and with increasing atomic number, the spectral lines merely shift towards higher frequencies.
He also gave the following relation \[\sqrt{\nu }=a\,(Z-b)\]
 where v = Frequency of emitted line, Z = Atomic number of target, a = Proportionality constant, b = Screening constant or Shielding constant.
(Z - b) = Effective atomic number
a and b doesn't depend on the nature of target. Different values of b are as follows
b = 1 for K-series
b = 7.4 for L-series
b = 19.2 for M-series
(1) Mosley's law supported Bohr's theory
(2) It experimentally determined the atomic number (Z) of elements.
(3) This law established the importance of ordering of elements in periodic table by atomic number and not by atomic weight.
(4) Gaps in Moseley's data for A = 43, 61, 72, 75 suggested existence of new elements which were later discovered.
(5) The atomic numbers of Cu, Ag and Pt were established to be 29, 47 and 78 respectively.
(6) When a vacancy occurs in the K-shell, there is still one electron remaining in the K-shell. An electron in the L-shell will feel an effective charge of (Z - 1)e due to + Ze from the nucleus and ? e from the remaining K-shell electron, because L-shell orbit is well outside the K-shell orbit.
(7) Wave length of characteristic spectrum \[\frac{1}{\lambda }=R{{(Z-b)}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\] and energy of X-ray radiations. \[\Delta E=h\nu =\frac{hc}{\lambda }=Rhc{{(Z-b)}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(8) If transition takes place from \[{{n}_{2}}=2\] to \[{{n}_{2}}=1\] (\[{{K}_{\alpha }}\]- line)
(i) \[a=\sqrt{\frac{3RC}{4}}=2.47\times {{10}^{15}}Hz\]
(ii) \[{{\nu }_{K\alpha }}=RC{{(Z-1)}^{2}}\left( 1-\frac{1}{{{2}^{2}}} \right)=\frac{3RC}{4}{{(Z-1)}^{2}}\] \[=2.47\times {{10}^{15}}{{(Z-1)}^{2}}Hz\]
(iii) In general the wavelength of all the K-lines are given by \[\frac{1}{{{\lambda }_{K}}}=R{{(Z-1)}^{2}}\,\left( 1-\frac{1}{{{n}^{2}}} \right)\] where n = 2, 3, 4, ?.
While for \[{{K}_{\alpha }}\] line \[{{\lambda }_{K\alpha }}=\frac{1216}{{{(Z-1)}^{2}}}{\AA}\]
(iv) \[{{E}_{K\alpha }}=10.2{{(Z-1)}^{2}}eV\]
where v = Frequency of emitted line, Z = Atomic number of target, a = Proportionality constant, b = Screening constant or Shielding constant.
(Z - b) = Effective atomic number
a and b doesn't depend on the nature of target. Different values of b are as follows
b = 1 for K-series
b = 7.4 for L-series
b = 19.2 for M-series
(1) Mosley's law supported Bohr's theory
(2) It experimentally determined the atomic number (Z) of elements.
(3) This law established the importance of ordering of elements in periodic table by atomic number and not by atomic weight.
(4) Gaps in Moseley's data for A = 43, 61, 72, 75 suggested existence of new elements which were later discovered.
(5) The atomic numbers of Cu, Ag and Pt were established to be 29, 47 and 78 respectively.
(6) When a vacancy occurs in the K-shell, there is still one electron remaining in the K-shell. An electron in the L-shell will feel an effective charge of (Z - 1)e due to + Ze from the nucleus and ? e from the remaining K-shell electron, because L-shell orbit is well outside the K-shell orbit.
(7) Wave length of characteristic spectrum \[\frac{1}{\lambda }=R{{(Z-b)}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\] and energy of X-ray radiations. \[\Delta E=h\nu =\frac{hc}{\lambda }=Rhc{{(Z-b)}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(8) If transition takes place from \[{{n}_{2}}=2\] to \[{{n}_{2}}=1\] (\[{{K}_{\alpha }}\]- line)
(i) \[a=\sqrt{\frac{3RC}{4}}=2.47\times {{10}^{15}}Hz\]
(ii) \[{{\nu }_{K\alpha }}=RC{{(Z-1)}^{2}}\left( 1-\frac{1}{{{2}^{2}}} \right)=\frac{3RC}{4}{{(Z-1)}^{2}}\] \[=2.47\times {{10}^{15}}{{(Z-1)}^{2}}Hz\]
(iii) In general the wavelength of all the K-lines are given by \[\frac{1}{{{\lambda }_{K}}}=R{{(Z-1)}^{2}}\,\left( 1-\frac{1}{{{n}^{2}}} \right)\] where n = 2, 3, 4, ?.
While for \[{{K}_{\alpha }}\] line \[{{\lambda }_{K\alpha }}=\frac{1216}{{{(Z-1)}^{2}}}{\AA}\]
(iv) \[{{E}_{K\alpha }}=10.2{{(Z-1)}^{2}}eV\] Current Affairs CategoriesArchive
Trending Current Affairs
You need to login to perform this action. |