 (1) Nuclear forces are short range forces. These do not exist at large distances greater than \[{{10}^{-15}}\,m\].
(2) Nuclear forces are the strongest forces in nature.
(3) These are attractive force and causes stability of the nucleus.
(4) These forces are charge independent.
(5) Nuclear forces are non-central force.
(6) Nuclear forces are exchange forces : According to scientist Yukawa the nuclear force between the two nucleons is the result of the exchange of particles called mesons between the nucleons.
\[\pi -\]mesons are of three types - Positive \[\pi \]meson \[({{\pi }^{+}})\], negative \[\pi \] meson \[({{\pi }^{-}})\], neutral \[\pi \] meson \[({{\pi }^{0}})\]
The force between neutron and proton is due to exchange of charged meson between them i.e.
\[p\to {{\pi }^{+}}+n,\,\,\,\,\,\,n\to p+{{\pi }^{-}}\]
The forces between a pair of neutrons or a pair of protons are the result of the exchange of neutral meson \[({{\pi }^{0}})\] between them i.e.
\[p\to p'+{{\pi }^{0}}\] and \[n\to n'+{{\pi }^{0}}\]
Thus exchange of p meson between nucleons keeps the nucleons bound together. It is responsible for the nuclear forces.
Dog-Bone analogy
The above interactions can be explained with the dog bone analogy according to which we consider the two interacting nucleons to be two dogs having a common bone clenched in between their teeth very firmly. Each one of these dogs wants to take the bone and hence they cannot be separated easily. They seem to be bound to each other with a strong attractive force (which is the bone) though the dogs themselves are strong enemies. The meson plays the same role of the common bone in between two nucleons.
(1) Nuclear forces are short range forces. These do not exist at large distances greater than \[{{10}^{-15}}\,m\].
(2) Nuclear forces are the strongest forces in nature.
(3) These are attractive force and causes stability of the nucleus.
(4) These forces are charge independent.
(5) Nuclear forces are non-central force.
(6) Nuclear forces are exchange forces : According to scientist Yukawa the nuclear force between the two nucleons is the result of the exchange of particles called mesons between the nucleons.
\[\pi -\]mesons are of three types - Positive \[\pi \]meson \[({{\pi }^{+}})\], negative \[\pi \] meson \[({{\pi }^{-}})\], neutral \[\pi \] meson \[({{\pi }^{0}})\]
The force between neutron and proton is due to exchange of charged meson between them i.e.
\[p\to {{\pi }^{+}}+n,\,\,\,\,\,\,n\to p+{{\pi }^{-}}\]
The forces between a pair of neutrons or a pair of protons are the result of the exchange of neutral meson \[({{\pi }^{0}})\] between them i.e.
\[p\to p'+{{\pi }^{0}}\] and \[n\to n'+{{\pi }^{0}}\]
Thus exchange of p meson between nucleons keeps the nucleons bound together. It is responsible for the nuclear forces.
Dog-Bone analogy
The above interactions can be explained with the dog bone analogy according to which we consider the two interacting nucleons to be two dogs having a common bone clenched in between their teeth very firmly. Each one of these dogs wants to take the bone and hence they cannot be separated easily. They seem to be bound to each other with a strong attractive force (which is the bone) though the dogs themselves are strong enemies. The meson plays the same role of the common bone in between two nucleons.

 (2) The stability or instability of a particular nucleus is determined by the competition between the attractive nuclear force among the protons and neutrons and the repulsive electrical interactions among the protons. Unstable nuclei decay, transforming themselves spontaneously into other structure by a variety of decay processes.
(3) We could not survive without the \[3.90\times {{10}^{26}}\] watt output of one near by fusion reactor, our sun.
(4) Nuclei are made up of proton and neutron. The number of protons in a nucleus (called the atomic number or proton number) is represented by the symbol Z. The number of neutrons (neutron number) is represented by N. The total number of neutrons and protons in a nucleus is called it's mass number A so \[A=Z+N\].
(5) Neutrons and proton, when described collectively are called nucleons. A single nuclear species having specific values of both Z and N is called a nuclide.
(6) Nuclides are represented as \[_{Z}{{X}^{A}};\] where X denotes the chemical symbol of the element.
(2) The stability or instability of a particular nucleus is determined by the competition between the attractive nuclear force among the protons and neutrons and the repulsive electrical interactions among the protons. Unstable nuclei decay, transforming themselves spontaneously into other structure by a variety of decay processes.
(3) We could not survive without the \[3.90\times {{10}^{26}}\] watt output of one near by fusion reactor, our sun.
(4) Nuclei are made up of proton and neutron. The number of protons in a nucleus (called the atomic number or proton number) is represented by the symbol Z. The number of neutrons (neutron number) is represented by N. The total number of neutrons and protons in a nucleus is called it's mass number A so \[A=Z+N\].
(5) Neutrons and proton, when described collectively are called nucleons. A single nuclear species having specific values of both Z and N is called a nuclide.
(6) Nuclides are represented as \[_{Z}{{X}^{A}};\] where X denotes the chemical symbol of the element. 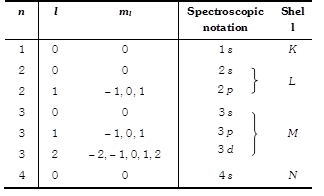
 The spectral lines arising from the transition of electron forms a spectra series.
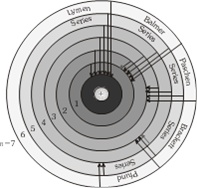
(1) Mainly there are five series and each series is named after it's discover as Lymen series, Balmer series, Paschen series, Bracket series and Pfund series.
(2) According to the Bohr's theory the wavelength of the radiations emitted from hydrogen atom is given by \[\frac{1}{\lambda }=R\,\left[ \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right]\]\[\Rightarrow \]\[\lambda =\frac{n_{1}^{2}n_{2}^{2}}{(n_{2}^{2}-n_{1}^{2})R}=\frac{n_{1}^{2}}{\left( 1-\frac{n_{1}^{2}}{n_{2}^{2}} \right)R}\]
where \[{{n}_{2}}=\]outer orbit (electron jumps from this orbit), \[{{n}_{1}}=\] inner orbit (electron falls in this orbit)
The spectral lines arising from the transition of electron forms a spectra series.
(1) Mainly there are five series and each series is named after it's discover as Lymen series, Balmer series, Paschen series, Bracket series and Pfund series.
(2) According to the Bohr's theory the wavelength of the radiations emitted from hydrogen atom is given by \[\frac{1}{\lambda }=R\,\left[ \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right]\]\[\Rightarrow \]\[\lambda =\frac{n_{1}^{2}n_{2}^{2}}{(n_{2}^{2}-n_{1}^{2})R}=\frac{n_{1}^{2}}{\left( 1-\frac{n_{1}^{2}}{n_{2}^{2}} \right)R}\]
where \[{{n}_{2}}=\]outer orbit (electron jumps from this orbit), \[{{n}_{1}}=\] inner orbit (electron falls in this orbit)
 (3) First line of the series is called first member, for this line wavelength is maximum \[({{\lambda }_{\max }})\]
For maximum wavelength if \[{{n}_{1}}=n\] then \[{{n}_{2}}=n+1\]
So \[{{\lambda }_{\max }}=\frac{{{n}^{2}}{{(n+1)}^{2}}}{(2n+1)R}\]
(4) Last line of the series is called series limit, for this line wavelength is minimum \[({{\lambda }_{\min }})\]
For minimum wavelength \[{{n}_{2}}=\infty ,\,{{n}_{1}}=n\] So \[{{\lambda }_{\min }}=\frac{{{n}^{2}}}{R}\]
(5) The ratio of first member and series limit can be calculated as \[\frac{{{\lambda }_{\max }}}{{{\lambda }_{\min }}}=\frac{{{(n+1)}^{2}}}{(2n+1)}\]
Different spectral series
(3) First line of the series is called first member, for this line wavelength is maximum \[({{\lambda }_{\max }})\]
For maximum wavelength if \[{{n}_{1}}=n\] then \[{{n}_{2}}=n+1\]
So \[{{\lambda }_{\max }}=\frac{{{n}^{2}}{{(n+1)}^{2}}}{(2n+1)R}\]
(4) Last line of the series is called series limit, for this line wavelength is minimum \[({{\lambda }_{\min }})\]
For minimum wavelength \[{{n}_{2}}=\infty ,\,{{n}_{1}}=n\] So \[{{\lambda }_{\min }}=\frac{{{n}^{2}}}{R}\]
(5) The ratio of first member and series limit can be calculated as \[\frac{{{\lambda }_{\max }}}{{{\lambda }_{\min }}}=\frac{{{(n+1)}^{2}}}{(2n+1)}\]
Different spectral series
| Spectral series | Transition | \[{{\lambda }_{\max }}\] | \[{{\lambda }_{\min }}\] | \[\frac{{{\lambda }_{max}}}{{{\lambda }_{min}}}\] | Region |
| 1. Lymen series | \[{{n}_{2}}=\] 2, 3, 4 ... \[\infty \] \[{{n}_{1}}=1\] | more...
When an electron makes transition from higher energy level having energy \[{{E}_{2}}({{n}_{2}})\] to a lower energy level having energy \[{{E}_{1}}({{n}_{1}})\] then a photon of frequency n is emitted
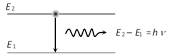 (1) Energy of emitted radiation
\[\Delta E={{E}_{2}}-{{E}_{1}}\]\[=\frac{-Rc\,h\,{{Z}^{2}}}{n_{2}^{2}}-\left( -\frac{Rch\,{{Z}^{2}}}{n_{1}^{2}} \right)\]
\[=13.6{{Z}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(2) Frequency of emitted radiation
\[\Delta E=h\nu \]\[\Rightarrow \]\[\nu =\frac{\Delta E}{h}=\frac{{{E}_{2}}-{{E}_{1}}}{h}=Rc\,{{Z}^{2}}\,\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(3) Wave number/wavelength
Wave number is the number of waves in unit length \[\bar{\nu }=\frac{1}{\lambda }=\frac{\nu }{c}\]\[\Rightarrow \frac{1}{\lambda }=R{{Z}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)=\frac{13.6{{Z}^{2}}}{hc}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(4) Number of spectral lines : If an electron jumps from higher energy orbit to lower energy orbit it emits raidations with various spectral lines.
If electron falls from orbit \[{{n}_{2}}\] to \[{{n}_{1}}\] then the number of spectral lines emitted is given by
\[{{N}_{E}}=\frac{({{n}_{2}}-{{n}_{1}}+1)({{n}_{2}}-{{n}_{1}})}{2}\]
If electron falls from \[{{n}^{th}}\] orbit to ground state (i.e. \[{{n}_{2}}=n\] and \[{{n}_{1}}=1\]) then number of spectral lines emitted \[{{N}_{E}}=\frac{n\,(n-1)}{2}\]
(5) Recoiling of an atom : Due to the transition of electron, photon is emitted and the atom is recoiled Recoil momentum of atom = momentum of photon \[=\frac{h}{\lambda }=hR{{Z}^{2}}\,\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
Also recoil energy of atom \[=\frac{{{p}^{2}}}{2m}\]\[=\frac{{{h}^{2}}}{2m{{\lambda }^{2}}}\] (where \[m=\] mass of recoil atom)
(1) Energy of emitted radiation
\[\Delta E={{E}_{2}}-{{E}_{1}}\]\[=\frac{-Rc\,h\,{{Z}^{2}}}{n_{2}^{2}}-\left( -\frac{Rch\,{{Z}^{2}}}{n_{1}^{2}} \right)\]
\[=13.6{{Z}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(2) Frequency of emitted radiation
\[\Delta E=h\nu \]\[\Rightarrow \]\[\nu =\frac{\Delta E}{h}=\frac{{{E}_{2}}-{{E}_{1}}}{h}=Rc\,{{Z}^{2}}\,\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(3) Wave number/wavelength
Wave number is the number of waves in unit length \[\bar{\nu }=\frac{1}{\lambda }=\frac{\nu }{c}\]\[\Rightarrow \frac{1}{\lambda }=R{{Z}^{2}}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)=\frac{13.6{{Z}^{2}}}{hc}\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
(4) Number of spectral lines : If an electron jumps from higher energy orbit to lower energy orbit it emits raidations with various spectral lines.
If electron falls from orbit \[{{n}_{2}}\] to \[{{n}_{1}}\] then the number of spectral lines emitted is given by
\[{{N}_{E}}=\frac{({{n}_{2}}-{{n}_{1}}+1)({{n}_{2}}-{{n}_{1}})}{2}\]
If electron falls from \[{{n}^{th}}\] orbit to ground state (i.e. \[{{n}_{2}}=n\] and \[{{n}_{1}}=1\]) then number of spectral lines emitted \[{{N}_{E}}=\frac{n\,(n-1)}{2}\]
(5) Recoiling of an atom : Due to the transition of electron, photon is emitted and the atom is recoiled Recoil momentum of atom = momentum of photon \[=\frac{h}{\lambda }=hR{{Z}^{2}}\,\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
Also recoil energy of atom \[=\frac{{{p}^{2}}}{2m}\]\[=\frac{{{h}^{2}}}{2m{{\lambda }^{2}}}\] (where \[m=\] mass of recoil atom) Current Affairs CategoriesArchive
Trending Current Affairs
You need to login to perform this action. |